ihtObtura: A novel liquid embolic agent with post-embolization radiopacity loss, in endovascular treatment of brain arteriovenous malformations, dural arteriovenous fistulas, and tumors: CLARIDAD trial
- PMID: 39019507
- PMCID: PMC12015003
- DOI: 10.1136/jnis-2023-021442
ihtObtura: A novel liquid embolic agent with post-embolization radiopacity loss, in endovascular treatment of brain arteriovenous malformations, dural arteriovenous fistulas, and tumors: CLARIDAD trial
Abstract
Background: Endovascular embolization is frequently used for vascular lesions of the head and neck. Newer agents may help to enhance visualization and improve treatment outcomes.
Methods: The CLARIDAD clinical trial was a prospective, single center, first-in-man investigation of neurovascular embolization using the novel embolic agent ihtObtura for a broad indication, covering the need for a liquid embolic agent in head and neck procedures. The primary outcomes assessed were therapeutic efficacy to deliver ihtObtura to embolize the catheterized pedicle and associated angiographic vascularity, and subsequent loss of radiopacity. Safety endpoints included procedural adverse events, modified Rankin Scale (mRS) score, morbidity, and mortality. Radiologic and clinical follow-up evaluations were conducted at 30, 90, 180 days, and 1 year post-treatment.
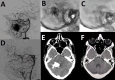
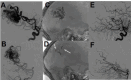
Results: 65 consecutive patients (mean age 37.8 years, 50.8% women) were treated over 129 sessions. A total of 42 brain arteriovenous malformations (AVMs; 90% grades III and IV), 8 dural arteriovenous fistulas (DAVFs), and 15 hypervascular tumors were treated with ihtObtura using an average of 3.9 mL per session and 7.7 mL per patient. We achieved therapeutic effectiveness in 99% of catheterizations. Radiopacity loss was complete after 74.3% of the sessions at 30 days, 95.6% at 90 days, and 100% at the 1 year follow-up. Serious adverse events (mRS score >2) occurred in two patients (3.1%) with previously ruptured high grade AVMs leading to one death and one permanent disabling morbidity.
Conclusions: The study showed that ihtObtura was a novel, safe, and effective liquid embolic agent for the treatment of AVMs, DAVFs, and hypervascular tumors. Its key property of significant radiopacity loss contributes to improve anatomical understanding, particularly in staged procedures, as well as reduction in post-procedural imaging artifact. There may be additional benefits of eliminating tantalum from the embolic mixture in terms of lesion penetration.
Keywords: Arteriovenous Malformation; Embolic; Fistula; Liquid Embolic Material; Tumor.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JCL reports consultancy for Iberhospitex and Medtronic. AG reports consultancy for Balt, Medtronic, and Iberhospitex. AHS reports Current Research Grants: Co-investigator for NIH - 1R01EB030092-01, Project Title: High Speed Angiography at 1000 frames per second; Mentor for Brain Aneurysm Foundation Carol W. Harvey Chair of Research, Sharon Epperson Chair of Research, Project Title: A Whole Blood RNA Diagnostic for Unruptured Brain Aneurysm: Risk Assessment Prototype Development and Testing Financial Interest/Investor/Stock Options/Ownership:Adona Medical, Inc., Basecamp Vascular SAS, Bend IT Technologies, Ltd., BlinkTBI, Inc, Borvo Medical, Inc., Cerebrotech Medical Systems, Inc., CerebrovaKP, Code Zero Medical, Inc., Cognition Medical, Collavidence, Inc., Contego Medical, Inc., CVAID Ltd., E8, Inc., Endostream Medical, Ltd, FreeOx Biotech, SL, Galaxy Therapeutics, Inc., Hyperion Surgical, Inc., Imperative Care, Inc., InspireMD, Ltd., Instylla, Inc., Launch NY, Inc., Neurolutions, Inc., NeuroRadial Technologies, Inc.(Sold to Medtronic in 2021), Neurovascular Diagnostics, Inc., Peijia Medical, PerFlow Medical, Ltd., Piraeus Medical, Inc., Q’Apel Medical, Inc., QAS.ai, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (Purchased 2019 by Integra Lifesciences, Corp), Rist Neurovascular, Inc. (Purchased 2020 by Medtronic), Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, Sim & Cure, Spinnaker Medical, Inc., StimMed, LLC, Synchron, Inc., Tulavi Therapeutics, Inc., Vastrax, LLC, Viseon, Inc., Whisper Medical, Inc., Willow Medtech, Inc. Consultant/Advisory Board:Amnis Therapeutics, Asahi Intecc Co Ltd., Boston Scientific, Canon Medical Systems USA, Inc., Cardinal Health 200, LLC, Cerebrotech Medical Systems, Inc., CerebrovaKP, Cerenovus, Contego Medical, Inc, Cordis, Corindus, Inc., Endostream Medical, Ltd, FreeOx Biotech, SL, Hyperfine Operations, Inc., Imperative Care, InspireMD, Ltd., Integra, IRRAS AB, Medtronic, MicroVention, Minnetronix Neuro, Inc., Peijia Medical, Penumbra, Piraeus Medical, Inc., Q’Apel Medical, Inc., Rapid Medical, Serenity Medical, Inc., Shockwave Medical, Inc., Silk Road Medical, StimMed, LLC, Stryker Neurovascular., Synchron Australia Pty Ltd., VasSol, Vesalio, Viz.ai, Inc., WL Gore National PI/Steering Committees:Cerenovus EXCELLENT and ARISE II Trial; Medtronic SWIFT PRIME, VANTAGE, EMBOLISE and SWIFT DIRECT Trials; MicroVention FRED Trial & CONFIDENCE Study; MUSC POSITIVE Trial; Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial, MIVI neuroscience EVAQ Trial; Rapid Medical SUCCESS Trial; InspireMD C-GUARDIANS IDE Pivotal Trial; Patents:Patent No. US 11,464,528 B2, Date: October 11, 2022, CLOT RETRIEVAL SYSTEM FOR REMOVING OCCLUSIVE CLOT FROM A BLOOD VESSEL, Applicant and Assignee: Neuravi Limited (Galway), Role: Co-Inventor.
Figures
Similar articles
-
ihtObtura®, a new liquid embolic agent for improving curative embolization of brain AVMs.AJNR Am J Neuroradiol. 2025 May 13:ajnr.A8834. doi: 10.3174/ajnr.A8834. Online ahead of print. AJNR Am J Neuroradiol. 2025. PMID: 40360184
-
LIQUID - Treatment of high-grade dural arteriovenous fistulas with Squid liquid embolic agent: a prospective, observational multicenter study.J Neurointerv Surg. 2023 Nov;15(11):1111-1116. doi: 10.1136/jnis-2022-019859. Epub 2023 Jan 6. J Neurointerv Surg. 2023. PMID: 36609544 Free PMC article.
-
Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas.Clin Neuroradiol. 2022 Mar;32(1):25-38. doi: 10.1007/s00062-021-01066-6. Epub 2021 Jul 29. Clin Neuroradiol. 2022. PMID: 34324005 Free PMC article. Review.
-
Transcirculation Approach for Endovascular Embolization of Intracranial Aneurysms, Arteriovenous Malformations, and Dural Fistulas: A Multicenter Study.World Neurosurg. 2020 Feb;134:e1015-e1027. doi: 10.1016/j.wneu.2019.11.078. Epub 2019 Nov 20. World Neurosurg. 2020. PMID: 31759150
-
Long-term follow-up of transarterial balloon-assisted Onyx embolization for endovascular treatment of dural arteriovenous fistulas: A single-institution case series and literature review.Clin Neurol Neurosurg. 2020 Dec;199:106256. doi: 10.1016/j.clineuro.2020.106256. Epub 2020 Oct 1. Clin Neurol Neurosurg. 2020. PMID: 33069089 Review.
Cited by
-
Comparing visualization performance of liquid embolic agents using a novel injectable phantom.Interv Neuroradiol. 2024 Aug 21:15910199241276581. doi: 10.1177/15910199241276581. Online ahead of print. Interv Neuroradiol. 2024. PMID: 39166277 Free PMC article.
References
-
- Jiang Y, Zhang Y, Lu Z, et al. Liquid embolic agents for interventional embolization. ChemPhysMater. 2022;1:39–50. doi: 10.1016/j.chphma.2021.09.008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
