Clinical impact of drug-drug interactions on abemaciclib in the real-world experience of AB-ITALY study
- PMID: 39019916
- PMCID: PMC11254918
- DOI: 10.1038/s41523-024-00657-z
Clinical impact of drug-drug interactions on abemaciclib in the real-world experience of AB-ITALY study
Erratum in
-
Author Correction: Clinical impact of drug-drug interactions on abemaciclib in the real-world experience of AB-ITALY study.NPJ Breast Cancer. 2024 Aug 2;10(1):68. doi: 10.1038/s41523-024-00682-y. NPJ Breast Cancer. 2024. PMID: 39095493 Free PMC article. No abstract available.
Abstract
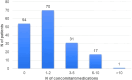
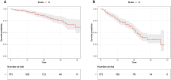
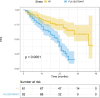
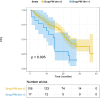
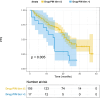
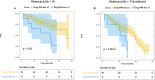
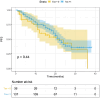
Abemaciclib demonstrated clinical benefit in women affected by HR+/HER2- advanced breast cancer (aBC). Drug-drug interactions (DDIs) can lead to reduced treatment efficacy or increased toxicity. This retro-prospective study aimed to evaluate outcomes, DDIs' impact, and toxicities of abemaciclib combined with endocrine therapy in a real-world setting. Patients from 12 referral Italian hospitals with HR+/HER2- aBC who received abemaciclib were included. Clinical data about comorbidities, concurrent medications, outcomes, and adverse events (AE) were collected. Drug-PIN® (Personalized Interactions Network) is a tool recognizing the role of multiple interactions between active and/or pro-drug forms combined with biochemical and demographic patient data. The software was used to define the Drug-PIN score and Drug-PIN tier (green, yellow, dark yellow, and red) for each patient. Univariate and multivariate analyses were performed to identify predictors of patients' PFS or toxicity. One hundred seventy-three patients were included. 13% of patients had >75years. The overall response rate (ORR) was 63%. The general population's median PFS (mPFS) was 22 months (mo), while mOS were not reached. Patients treated with abemaciclib in combination with AI and fulvestrant had a mPFS of 36 and 19 mo, respectively. The most common toxicities were diarrhea, asthenia, and neutropenia detected in 63%,49%, and 49% of patients. The number of concomitant medications and comorbidities were not associated with survival outcomes (22 vs 17 mo, p = 0.068, p = 0.99). Drug-PIN tier from dark yellow to red and Drug-PIN score >12 were associated with shorter PFS compared to no/low-risk DDIs and score <12 (15 vs 23, p = 0.005, p = 0.0017). Drug interaction was confirmed as an independent biomarker in a multivariate model (p = 0.02). No difference in any grade AE, severe toxicities, and diarrhea were detected among different age subgroups. No association was found between Drug-PIN score or Drug-PIN tier and overall toxicity (p = 0.44), severe AEs (p = 0.11), or drug reduction (p = 0.27). The efficacy and safety of abemaciclib plus ET were confirmed in a real-world setting, even in the elderly population and patients with comorbidities. Evaluation of DDIs with Drug-PIN appears to be an independent predictor of PFS.
© 2024. The Author(s).
Conflict of interest statement
Simone Scagnoli (SS) speaker: novartis, pfizer, roche, lilly, BMS, MSD; Simona Pisegna (SP) speaker for Novartis, Lilly, Pfizer, Roche; Angela Toss (AT) speaker and advisory board Lilly, Novartis, Pfizer, AstraZeneca, MSD; Roberta Caputo (RC) advisory role Roche, Lilly, Novartis, MSD, Gilead, Daiichi Sankyo, Pierre-Fabre; Michelino De Laurentis (MdL) consultation for astrazeneca, amgen, celgene, daiichi sankyo, EIsai, Eli lilly, Exact science, Gilead, MSD, novartis, pfizer, pierre fabre, roche, seagen; Michela Palleschi (MP) advisory for Novartis and Lilly; Ugo de Giorgi (UdG) advisory Merck, BMS, Janssen, astellas, sanofi, bayer, pfizer, ipsen, novartis, ; grants: astrazeneca, sanofi, roche; Enrico Cortesi (EC) honoraria from MSD and astellas, advisory role for astellas, bayer; research found from merck; Agnese Fabbri (AF) advisory and speaker for lilly, pfizer, roche, novartis, gilead; Alessandra Fabi (AF) advisory board roche, pfizer, novartis, gilead, sophos, Seagen, Astrazeneca, lilly, pierre Fabre, exact science; Ida Paris (IP) fee for Novartis, Gentili, Italfarmaco, Genetic, advisory role for Gilead, AstraZeneca, Lilly, Pfizer, Seagen; Armando Orlandi (AO) fee speaker and advisory: amgen, lilly, pfizer, novartis, daiichi sankyo, gilead; Giuseppe Curigliano (GC) has/had a consultant/advisory role for Roche-Genentech, MSD, Pfizer, BMS, Amgen, Novartis, Pierre Fabre, Gilead, Eli-Lilly, Seagen, Exact Science; Carmen Criscitiello (CC) had consultancy/advisory role/speaker bureau Pfizer Roche msd Novartis Lilly seagen gilead Daiichi-Sankyo; Giuliana D’Auria (GdA) advisory role for amgen, pfizer, lilly, novartis, eisai; Patrizia Vici (PV) advisory role for Pfizer, lilly, eisai, Novartis; Alessio Cirillo (AC) speaker for Novartis, MSD; Daniele Santini (DS) advisory role for Angen, Janssen, Astellas, Bayer, Servier, Novartis, MSD. Merck, Pfizer, Ipsen; Paolo Marchetti (PM) has/had a consultant/advisory role for BMS, Roche, Genentech, MSD, Novartis, Amgen, Merck Serono, Pierre-Fabre, and Incyte. Andrea Botticelli (AB) has/had a consultant/advisory role for Roche-Genentech, MSD, Pfizer, BMS, Amgen, Novartis, Pierre Fabre, Gilead, Eli-Lilly, and Seagen. Competing interests: Maurizio Simmaco (MS), Paolo Marchetti, and Robert Preissner (RP) are members of the Advisory Board of Drug-PIN AG (software expressly cited in the text). The Drug-PIN AG is the holder of the patent PCT/IB2019/052310. The remaining authors declare no competing interests.
Figures

References
-
- Sledge, G. W. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35 (2017). - PubMed
-
- Goetz, M. P. et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 10.1200/JCO.2017.75.6155 (2017). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

