Selective pressures of platinum compounds shape the evolution of therapy-related myeloid neoplasms
- PMID: 39019934
- PMCID: PMC11255340
- DOI: 10.1038/s41467-024-50384-z
Selective pressures of platinum compounds shape the evolution of therapy-related myeloid neoplasms
Abstract
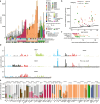
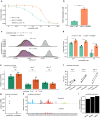
Therapy-related myeloid neoplasms (t-MN) arise as a complication of chemo- and/or radiotherapy. Although t-MN can occur both in adult and childhood cancer survivors, the mechanisms driving therapy-related leukemogenesis likely vary across different ages. Chemotherapy is thought to induce driver mutations in children, whereas in adults pre-existing mutant clones are selected by the exposure. However, selective pressures induced by chemotherapy early in life are less well studied. Here, we use single-cell whole genome sequencing and phylogenetic inference to show that the founding cell of t-MN in children starts expanding after cessation of platinum exposure. In patients with Li-Fraumeni syndrome, characterized by a germline TP53 mutation, we find that the t-MN already expands during treatment, suggesting that platinum-induced growth inhibition is TP53-dependent. Our results demonstrate that germline aberrations can interact with treatment exposures in inducing t-MN, which is important for the development of more targeted, patient-specific treatment regimens and follow-up.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

