Inhibition of IL-11 signalling extends mammalian healthspan and lifespan
- PMID: 39020175
- PMCID: PMC11291288
- DOI: 10.1038/s41586-024-07701-9
Inhibition of IL-11 signalling extends mammalian healthspan and lifespan
Abstract
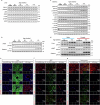
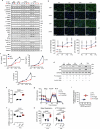
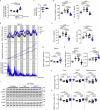
For healthspan and lifespan, ERK, AMPK and mTORC1 represent critical pathways and inflammation is a centrally important hallmark1-7. Here we examined whether IL-11, a pro-inflammatory cytokine of the IL-6 family, has a negative effect on age-associated disease and lifespan. As mice age, IL-11 is upregulated across cell types and tissues to regulate an ERK-AMPK-mTORC1 axis to modulate cellular, tissue- and organismal-level ageing pathologies. Deletion of Il11 or Il11ra1 protects against metabolic decline, multi-morbidity and frailty in old age. Administration of anti-IL-11 to 75-week-old mice for 25 weeks improves metabolism and muscle function, and reduces ageing biomarkers and frailty across sexes. In lifespan studies, genetic deletion of Il11 extended the lives of mice of both sexes, by 24.9% on average. Treatment with anti-IL-11 from 75 weeks of age until death extends the median lifespan of male mice by 22.5% and of female mice by 25%. Together, these results demonstrate a role for the pro-inflammatory factor IL-11 in mammalian healthspan and lifespan. We suggest that anti-IL-11 therapy, which is currently in early-stage clinical trials for fibrotic lung disease, may provide a translational opportunity to determine the effects of IL-11 inhibition on ageing pathologies in older people.
© 2024. The Author(s).
Conflict of interest statement
A.A.W., B.C., B.K.S., S.S. and S.A.C. are co-inventors of a patent family that includes: WO2022090509A (methods to extend healthspan and treat age-related diseases) and WO2018109174 (IL-11 antibodies). S.S. and S.A.C. are co-founders and shareholders of Enleofen Bio Pte Ltd and VVB Bio Pte Ltd. A.A.W. had consulted for VVB Bio on work unrelated to the study presented here. J.G. has acted as a consultant for Unity Biotechnology, Geras Bio, Myricx Pharma and Merck KGaA. Pfizer and Unity Biotechnology have funded research in the J.G. laboratory unrelated to the work presented here. J.G. owns equity in Geras Bio. J.G. is a named inventor in MRC and Imperial College patents related to senolytic therapies (the patents are not related to the work presented here). The other authors declare no competing interests.
Figures












Comment in
-
Anti-IL-11 antibody shows anti-ageing properties.Nat Rev Drug Discov. 2024 Sep;23(9):658. doi: 10.1038/d41573-024-00132-1. Nat Rev Drug Discov. 2024. PMID: 39090176 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

