Symbolic recording of signalling and cis-regulatory element activity to DNA
- PMID: 39020177
- PMCID: PMC11357993
- DOI: 10.1038/s41586-024-07706-4
Symbolic recording of signalling and cis-regulatory element activity to DNA
Abstract
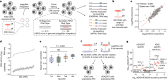
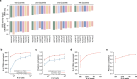
Measurements of gene expression or signal transduction activity are conventionally performed using methods that require either the destruction or live imaging of a biological sample within the timeframe of interest. Here we demonstrate an alternative paradigm in which such biological activities are stably recorded to the genome. Enhancer-driven genomic recording of transcriptional activity in multiplex (ENGRAM) is based on the signal-dependent production of prime editing guide RNAs that mediate the insertion of signal-specific barcodes (symbols) into a genomically encoded recording unit. We show how this strategy can be used for multiplex recording of the cell-type-specific activities of dozens to hundreds of cis-regulatory elements with high fidelity, sensitivity and reproducibility. Leveraging signal transduction pathway-responsive cis-regulatory elements, we also demonstrate time- and concentration-dependent genomic recording of WNT, NF-κB and Tet-On activities. By coupling ENGRAM to sequential genome editing via DNA Typewriter1, we stably record information about the temporal dynamics of two orthogonal signalling pathways to genomic DNA. Finally we apply ENGRAM to integratively record the transient activity of nearly 100 transcription factor consensus motifs across daily windows spanning the differentiation of mouse embryonic stem cells into gastruloids, an in vitro model of early mammalian development. Although these are proof-of-concept experiments and much work remains to fully realize the possibilities, the symbolic recording of biological signals or states within cells, to the genome and over time, has broad potential to complement contemporary paradigms for how we make measurements in biological systems.
© 2024. The Author(s).
Conflict of interest statement
The University of Washington has filed a patent application partially based on this work, in which J.C., W.C. and J.S. are listed as inventors. J.S. is on the scientific advisory board, a consultant and/or a cofounder of Adaptive Biotechnologies, Cajal Neuroscience, Camp4 Therapeutics, Guardant Health, Maze Therapeutics, Pacific Biosciences, Phase Genomics, Prime Medicine, Scale Biosciences, Somite Therapeutics and Sixth Street Capital. The remaining authors declare no competing interests.
Figures













References
-
- Golic, K. G. & Lindquist, S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell59, 499–509 (1989). - PubMed
-
- Kretzschmar, K. & Watt, F. M. Lineage tracing. Cell148, 33–45 (2012). - PubMed
-
- Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature450, 56–62 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

