Foetal gluten immunogenic peptides during pregnancy: a new determinant on the coeliac exposome
- PMID: 39020299
- PMCID: PMC11256569
- DOI: 10.1186/s12916-024-03495-9
Foetal gluten immunogenic peptides during pregnancy: a new determinant on the coeliac exposome
Abstract
Background: The increasing incidence of coeliac disease is leading to a growing interest in active search for associated factors, even the intrauterine and early life. The exposome approach to disease encompasses a life course perspective from conception onwards has recently been highlighted. Knowledge of early exposure to gluten immunogenic peptides (GIP) in utero could challenge the chronology of early prenatal tolerance or inflammation, rather than after the infant's solid diet after birth.
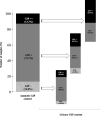
Methods: We developed an accurate and specific immunoassay to detect GIP in amniotic fluid (AF) and studied their accumulates, excretion dynamics and foetal exposure resulting from AF swallowing. One hundred twenty-five pregnant women with different gluten diets and gestational ages were recruited.
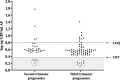
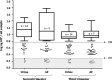
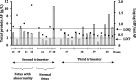
Results: GIP were detectable in AF from at least the 16th gestational week in gluten-consuming women. Although no significant differences in GIP levels were observed during gestation, amniotic GIP late pregnancy was not altered by maternal fasting, suggesting closed-loop entailing foetal swallowing of GIP-containing AF and subsequent excretion via the foetal kidneys.
Conclusions: The study shows evidence, for the first time, of the foetal exposure to gluten immunogenic peptides and establishes a positive correlation with maternal gluten intake. The results obtained point to a novel physiological concept as they describe a plausible closed-loop circuit entailing foetal swallowing of GIP contained in AF and its subsequent excretion through the foetal kidneys. The study adds important new information to understanding the coeliac exposome.
Keywords: Coeliac disease; Exposome; Gluten; Gluten immunogenic peptides.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

