Biological characteristics of tissue engineered-nerve grafts enhancing peripheral nerve regeneration
- PMID: 39020413
- PMCID: PMC11256578
- DOI: 10.1186/s13287-024-03827-9
Biological characteristics of tissue engineered-nerve grafts enhancing peripheral nerve regeneration
Abstract
Background: A favorable regenerative microenvironment is essential for peripheral nerve regeneration. Neural tissue-specific extracellular matrix (ECM) is a natural material that helps direct cell behavior and promote axon regeneration. Both bone marrow-derived mesenchymal stem cells (BMSCs) and adipose-derived mesenchymal stem cells (ADSCs) transplantation are effective in repairing peripheral nerve injury (PNI). However, there is no study that characterizes the in vivo microenvironmental characteristics of these two MSCs for the early repair of PNI when combined with neural tissue-derived ECM materials, i.e., acellular nerve allograft (ANA).
Methods: In order to investigate biological characteristics, molecular mechanisms of early stage, and effectiveness of ADSCs- or BMSCs-injected into ANA for repairing PNI in vivo, a rat 10 mm long sciatic nerve defect model was used. We isolated primary BMSCs and ADSCs from bone marrow and adipose tissue, respectively. First, to investigate the in vivo response characteristics and underlying molecular mechanisms of ANA combined with BMSCs or ADSCs, eighty-four rats were randomly divided into three groups: ANA group, ANA+BMSC group, and ANA+ADSC group. We performed flow cytometry, RT-PCR, and immunofluorescence staining up to 4 weeks postoperatively. To further elucidate the underlying molecular mechanisms, changes in long noncoding RNAs (lncRNAs), circular RNAs (circRNAs), microRNAs (miRNAs), and messenger RNAs (mRNAs) were systematically investigated using whole transcriptome sequencing. We then constructed protein-protein interaction networks to find 10 top ranked hub genes among differentially expressed mRNAs. Second, in order to explore the effectiveness of BMSCs and ADSCs on neural tissue-derived ECM materials for repairing PNI, sixty-eight rats were randomized into four groups: ANA group, ANA+BMSC group, ANA+ADSC group, and AUTO group. In the ANA+BMSC and ANA+ADSC groups, ADSCs/BMSCs were equally injected along the long axis of the 10-mm ANA. Then, we performed histological and functional assessments up to 12 weeks postoperatively.
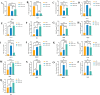
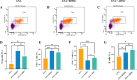
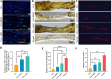
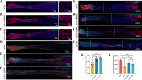
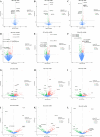
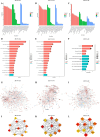
Results: The results of flow cytometry and RT-PCR showed that ANA combined with BMSCs exhibited more significant immunomodulatory effects, as evidenced by the up-regulation of interleukin (IL)-10, down-regulation of IL-1β and tumor necrosis factor-alpha (TNF-α) expression, promotion of M1-type macrophage polarization to M2-type, and a significant increase in the number of regulatory T cells (Tregs). ANA combined with ADSCs exhibited more pronounced features of pro-myelination and angiogenesis, as evidenced by the up-regulation of myelin-associated protein gene (MBP and MPZ) and angiogenesis-related factors (TGF-β, VEGF). Moreover, differentially expressed genes from whole transcriptome sequencing results further indicated that ANA loaded with BMSCs exhibited notable immunomodulatory effects and ANA loaded with ADSCs was more associated with angiogenesis, axonal growth, and myelin formation. Notably, ANA infused with BMSCs or ADSCs enhanced peripheral nerve regeneration and motor function recovery with no statistically significant differences.
Conclusions: This study revealed that both ANA combined with BMSCs and ADSCs enhance peripheral nerve regeneration and motor function recovery, but their biological characteristics (mainly including immunomodulatory effects, pro-vascular regenerative effects, and pro-myelin regenerative effects) and underlying molecular mechanisms in the process of repairing PNI in vivo are different, providing new insights into MSC therapy for peripheral nerve injury and its clinical translation.
Keywords: Extracellular matrix; Mesenchymal stem cells; Peripheral nerve injury; Vascular regeneration; Whole transcriptome sequencing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Frostick SP. The physiological and metabolic consequences of muscle denervation. Int Angiol. 1995;14:278–287. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

