Atezolizumab combined with immunogenic salvage chemoimmunotherapy in patients with transformed diffuse large B-cell lymphoma
- PMID: 39021209
- PMCID: PMC11694104
- DOI: 10.3324/haematol.2024.285185
Atezolizumab combined with immunogenic salvage chemoimmunotherapy in patients with transformed diffuse large B-cell lymphoma
Abstract
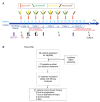
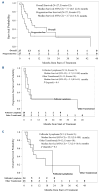
Patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) transformed from indolent B-cell lymphomas, including Richter transformation, have a poor prognosis. PD-1/PD-L1 antibodies produce modest objective and complete response rates in B-cell non-Hodgkin lymphoma as monotherapy but may synergize with immunogenic chemotherapies such as gemcitabine and oxaliplatin (GemOx). Thus, we evaluated the safety and efficacy of atezolizumab plus rituximab and GemOx (R-GemOx+Atezo) in R/R transformed DLBCL, including Richter transformation. We conducted a phase I trial including patients with transformed DLBCL after ≥1 prior therapy. Patients received up to four cycles of R-GemOx+Atezo. Patients in complete remission could then proceed to R-Atezo maintenance until progression. A safety lead-in with evaluation of dose-limiting toxicity was performed to confirm the recommended phase II dose; subsequently the treatment was administered to two expansion cohorts: one with transformed follicular lymphoma (FL) and the other with non-FL transformed DLBCL, including Richter transformation. Twenty-seven patients were enrolled. One of the six patients in the safety lead-in had a dose-limiting toxicity attributed to atezolizumab, a grade 4 Stevens-Johnson syndrome. The most common grade ≥3 events were neutropenia (18.5%), lymphopenia (18.5%), and thrombocytopenia (14.8%). The overall and complete response rates were 59% and 33%, respectively. The overall and complete response rates in transformed FL were 79% and 43%, respectively, and 38% and 23% in transformed non-FL, respectively. The median progression-free survival and overall survival of the total population were 4.2 and 7.7 months, respectively. R-GemOx+Atezo was well tolerated and demonstrated promising preliminary efficacy in patients with relapsed/refractory transformed DLBCL.
Figures

References
-
- Kuruvilla J, MacDonald DA, Kouroukis CT, et al. Salvage chemotherapy and autologous stem cell transplantation for transformed indolent lymphoma: a subset analysis of NCIC CTG LY12. Blood. 2015;126(6):733-738. - PubMed
-
- Zelenetz AD, Gordon LI, Chang JE, et al. NCCN Guidelines® insights: B-cell lymphomas, version 5.2021. J Natl Compr Canc Netw. 2021;19(11):1218-1230. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

