Reducing stomatal density by expression of a synthetic epidermal patterning factor increases leaf intrinsic water use efficiency and reduces plant water use in a C4 crop
- PMID: 39021331
- PMCID: PMC11565208
- DOI: 10.1093/jxb/erae289
Reducing stomatal density by expression of a synthetic epidermal patterning factor increases leaf intrinsic water use efficiency and reduces plant water use in a C4 crop
Abstract
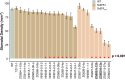
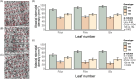
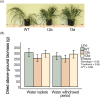
Enhancing crop water use efficiency (WUE) is a key target trait for climatic resilience and expanding cultivation on marginal lands. Engineering lower stomatal density to reduce stomatal conductance (gs) has improved WUE in multiple C3 crop species. However, reducing gs in C3 species often reduces photosynthetic carbon gain. A different response is expected in C4 plants because they possess specialized anatomy and biochemistry which concentrates CO2 at the site of fixation. This modifies the relationship of photosynthesis (AN) with intracellular CO2 concentration (ci), such that photosynthesis is CO2 saturated and reductions in gs are unlikely to limit AN. To test this hypothesis, genetic strategies were investigated to reduce stomatal density in the C4 crop sorghum. Constitutive expression of a synthetic epidermal patterning factor (EPF) transgenic allele in sorghum led to reduced stomatal densities, reduced gs, reduced plant water use, and avoidance of stress during a period of water deprivation. In addition, moderate reduction in stomatal density did not increase stomatal limitation to AN. However, these positive outcomes were associated with negative pleiotropic effects on reproductive development and photosynthetic capacity. Avoiding pleiotropy by targeting expression of the transgene to specific tissues could provide a pathway to improved agronomic outcomes.
Keywords: Sorghum bicolor; C4 photosynthesis; stomata; water-use efficiency.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Bioenergy sorghum maintains photosynthetic capacity in elevated ozone concentrations.Plant Cell Environ. 2021 Mar;44(3):729-746. doi: 10.1111/pce.13962. Epub 2021 Jan 21. Plant Cell Environ. 2021. PMID: 33245145 Free PMC article.
-
Fast stomatal kinetics in sorghum enable tight coordination with photosynthetic responses to dynamic light intensity and safeguard high water use efficiency.J Exp Bot. 2024 Nov 15;75(21):6796-6809. doi: 10.1093/jxb/erae389. J Exp Bot. 2024. PMID: 39292501 Free PMC article.
-
Increasing water use efficiency along the C3 to C4 evolutionary pathway: a stomatal optimization perspective.J Exp Bot. 2014 Jul;65(13):3683-93. doi: 10.1093/jxb/eru205. Epub 2014 May 23. J Exp Bot. 2014. PMID: 24860185 Free PMC article.
-
Water Use Efficiency as a Constraint and Target for Improving the Resilience and Productivity of C3 and C4 Crops.Annu Rev Plant Biol. 2019 Apr 29;70:781-808. doi: 10.1146/annurev-arplant-042817-040305. Annu Rev Plant Biol. 2019. PMID: 31035829 Review.
-
Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel.Proc Biol Sci. 2009 Jul 7;276(1666):2333-43. doi: 10.1098/rspb.2008.1517. Epub 2009 Feb 25. Proc Biol Sci. 2009. PMID: 19324804 Free PMC article. Review.
Cited by
-
StEPF2 and StEPFL9 Play Opposing Roles in Regulating Stomatal Development and Drought Tolerance in Potato (Solanum tuberosum L.).Int J Mol Sci. 2024 Oct 5;25(19):10738. doi: 10.3390/ijms251910738. Int J Mol Sci. 2024. PMID: 39409067 Free PMC article.
-
Stomata: custodians of leaf gaseous exchange.J Exp Bot. 2024 Nov 15;75(21):6677-6682. doi: 10.1093/jxb/erae425. J Exp Bot. 2024. PMID: 39545386 Free PMC article. No abstract available.
-
Greater aperture counteracts effects of reduced stomatal density on water use efficiency: a case study on sugarcane and meta-analysis.J Exp Bot. 2024 Nov 15;75(21):6837-6849. doi: 10.1093/jxb/erae271. J Exp Bot. 2024. PMID: 39021256 Free PMC article.
-
Challenges of translating Arabidopsis insights into crops.Plant Cell. 2025 May 9;37(5):koaf059. doi: 10.1093/plcell/koaf059. Plant Cell. 2025. PMID: 40178150 Free PMC article. Review.
-
Advancements in Water-Saving Strategies and Crop Adaptation to Drought: A Comprehensive Review.Physiol Plant. 2025 Jul-Aug;177(4):e70332. doi: 10.1111/ppl.70332. Physiol Plant. 2025. PMID: 40599019 Free PMC article. Review.
References
-
- Abràmoff MD, Magalhães PJ, Ram SJ.. 2006. Image processing with ImageJ. In: Cox G, ed. Optical imaging techniques in cell biology. Boca Raton, FL: CRC Press, 249–258.
-
- Blum A. 2005. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research 56, 1159–1168.
-
- Boyer JS. 1982. Plant productivity and environment. Science 218, 443–448. - PubMed
-
- Briggs L, Shantz H.. 1917. The water requirement of plants as influenced by environment. In: Proceedings of the Second Pan-American Science Congress. Washington DC, 95–107.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

