Hyaluronic acid modified indocyanine green nanoparticles: a novel targeted strategy for NIR-II fluorescence lymphatic imaging
- PMID: 39021390
- PMCID: PMC11251975
- DOI: 10.3389/fchem.2024.1435627
Hyaluronic acid modified indocyanine green nanoparticles: a novel targeted strategy for NIR-II fluorescence lymphatic imaging
Abstract
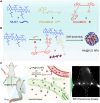
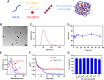
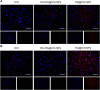
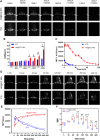
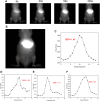
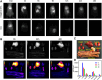
The lymphatic system, alongside blood circulation, is crucial for maintaining bodily equilibrium and immune surveillance. Despite its importance, lymphatic imaging techniques lag behind those for blood circulation. Fluorescence imaging, particularly in the near-infrared-II (NIR-II) region, offers promising capabilities with centimeter-scale tissue penetration and micron-scale spatial resolution, sparking interest in visualizing the lymphatic system. Although indocyanine green (ICG) has been approved by the Food and Drug Administration (FDA) for use as a near-infrared-I (NIR-I) region fluorescent dye, its limitations include shallow penetration depth and low signal-to-noise ratio. Research suggests that ICG's fluorescence emission tail in the second near-infrared window holds potential for high-quality NIR-II imaging. However, challenges like short circulation half-life and concentration-dependent aggregation hinder its wider application. Here we developed HA@ICG nanoparticles (NPs), a superior ICG-based NIR-II fluorescent probe with excellent biocompatibility, prolonging in vivo imaging, and enhancing photostability compared to ICG alone. Leveraging LYVE-1, a prominent lymphatic endothelial cell receptor that binds specifically to hyaluronic acid (HA), our nanoprobes exhibit exceptional performance in targeting lymphatic system imaging. Moreover, our findings demonstrate the capability of HA@ICG NPs for capillary imaging, offering a means to assess local microcirculatory blood supply. These compelling results underscore the promising potential of HA@ICG NPs for achieving high-resolution bioimaging of nanomedicines in the NIR-II window.
Keywords: LYVE-1; NIR-II imaging; fluorescence imaging; hyaluronic acid; indocyanine green; lymphatic imaging.
Copyright © 2024 Zhang, Wang, Zhang, Ma, Qi, Du and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

