Association of connexin36 with adherens junctions at mixed synapses and distinguishing electrophysiological features of those at mossy fiber terminals in rat ventral hippocampus
- PMID: 39021415
- PMCID: PMC11249852
- DOI: 10.62347/RTMH4490
Association of connexin36 with adherens junctions at mixed synapses and distinguishing electrophysiological features of those at mossy fiber terminals in rat ventral hippocampus
Abstract
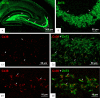
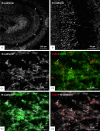
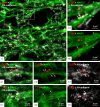
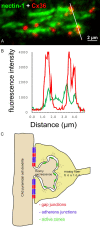
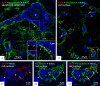
Background: Granule cells in the hippocampus project axons to hippocampal CA3 pyramidal cells where they form large mossy fiber terminals. We have reported that these terminals contain the gap junction protein connexin36 (Cx36) specifically in the stratum lucidum of rat ventral hippocampus, thus creating morphologically mixed synapses that have the potential for dual chemical/electrical transmission.
Methodology: Here, we used various approaches to characterize molecular and electrophysiological relationships between the Cx36-containing gap junctions at mossy fiber terminals and their postsynaptic elements and to examine molecular relationships at mixed synapses in the brainstem.
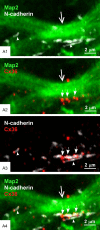
Results: In rat and human ventral hippocampus, many of these terminals, identified by their selective expression of vesicular zinc transporter-3 (ZnT3), displayed multiple, immunofluorescent Cx36-puncta representing gap junctions, which were absent at mossy fiber terminals in the dorsal hippocampus. In rat, these were found in close proximity to the protein constituents of adherens junctions (i.e., N-cadherin and nectin-1) that are structural hallmarks of mossy fiber terminals, linking these terminals to the dendritic shafts of CA3 pyramidal cells, thus indicating the loci of gap junctions at these contacts. Cx36-puncta were also associated with adherens junctions at mixed synapses in the brainstem, supporting emerging views of the structural organization of the adherens junction-neuronal gap junction complex. Electrophysiologically induced long-term potentiation (LTP) of field responses evoked by mossy fiber stimulation was greater in the ventral than dorsal hippocampus.
Conclusions: The electrical component of transmission at mossy fiber terminals may contribute to enhanced LTP responses in the ventral hippocampus.
Keywords: Gap junctions; brainstem; electrical coupling; hippocampus; immunofluorescence; long-term potentiation; mixed chemical/electrical synapses; neurotransmission.
IJPPP Copyright © 2024.
Conflict of interest statement
None.
Figures






References
-
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. - PubMed
-
- Connors BW. Electrical signaling with neuronal gap junctions. In: Harris A, Locke D, editors. Connexins: A Guide. Springer: Humana Press; 2009. pp. 143–164.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
