Active prokaryotic and eukaryotic viral ecology across spatial scale in a deep-sea brine pool
- PMID: 39021441
- PMCID: PMC11252502
- DOI: 10.1093/ismeco/ycae084
Active prokaryotic and eukaryotic viral ecology across spatial scale in a deep-sea brine pool
Abstract
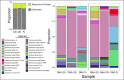
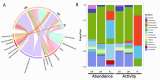
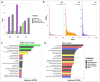
Deep-sea brine pools represent rare, extreme environments, providing unique insight into the limits of life on Earth, and by analogy, the plausibility of life beyond it. A distinguishing feature of many brine pools is presence of thick microbial mats that develop at the brine-seawater interface. While these bacterial and archaeal communities have received moderate attention, viruses and their host interactions in these environments remain underexplored. To bridge this knowledge gap, we leveraged metagenomic and metatranscriptomic data from three distinct zones within the NEOM brine pool system (Gulf of Aqaba) to reveal the active viral ecology around the pools. We report a remarkable diversity and activity of viruses infecting microbial hosts in this environment, including giant viruses, RNA viruses, jumbo phages, and Polinton-like viruses. Many of these form distinct clades-suggesting presence of untapped viral diversity in this ecosystem. Brine pool viral communities exhibit zone-specific differences in infection strategy-with lysogeny dominating the bacterial mat further away from the pool's center. We linked viruses to metabolically important prokaryotes-including association between a jumbo phage and a key manganese-oxidizing and arsenic-metabolizing bacterium. These foundational results illuminate the role of viruses in modulating brine pool microbial communities and biogeochemistry through revealing novel viral diversity, host associations, and spatial heterogeneity in viral dynamics.
Keywords: NEOM brine pools; brine pool viruses; deep-sea viruses; marine viral diversity; marine virus ecology; virus–host interactions.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Anschutz P, Blanc G, Chatin Fet al. Hydrographic changes during 20 years in the brine-filled basins of the Red Sea. Deep-Sea Res I Oceanogr Res Pap 1999;46:1779–92. 10.1016/S0967-0637(99)00019-9 - DOI
-
- Schardt C. Hydrothermal fluid migration and brine pool formation in the Red Sea: the Atlantis II deep. Mineral Deposita 2016;51:89–111. 10.1007/s00126-015-0583-2 - DOI
-
- Ross DA, Hunt JM. Third brine pool in the Red Sea. Nature 1967;213:687–8. 10.1038/213687a0 - DOI
-
- Backer H, Schoell M. New deeps with brines and metalliferous sediments in the Red Sea. Nat Phys Sci 1972;240:153–8. 10.1038/physci240153a0 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

