Clinical criteria for a limbic-predominant amnestic neurodegenerative syndrome
- PMID: 39021510
- PMCID: PMC11251771
- DOI: 10.1093/braincomms/fcae183
Clinical criteria for a limbic-predominant amnestic neurodegenerative syndrome
Abstract
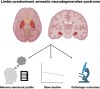
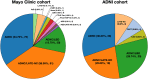
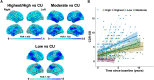
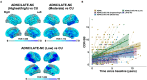
Predominant limbic degeneration has been associated with various underlying aetiologies and an older age, predominant impairment of episodic memory and slow clinical progression. However, the neurological syndrome associated with predominant limbic degeneration is not defined. This endeavour is critical to distinguish such a syndrome from those originating from neocortical degeneration, which may differ in underlying aetiology, disease course and therapeutic needs. We propose a set of clinical criteria for a limbic-predominant amnestic neurodegenerative syndrome that is highly associated with limbic-predominant age-related TDP-43 encephalopathy but also other pathologic entities. The criteria incorporate core, standard and advanced features, including older age at evaluation, mild clinical syndrome, disproportionate hippocampal atrophy, impaired semantic memory, limbic hypometabolism, absence of neocortical degeneration and low likelihood of neocortical tau, with degrees of certainty (highest, high, moderate and low). We operationalized this set of criteria using clinical, imaging and biomarker data to validate its associations with clinical and pathologic outcomes. We screened autopsied patients from Mayo Clinic and Alzheimer's Disease Neuroimaging Initiative cohorts and applied the criteria to those with an antemortem predominant amnestic syndrome (Mayo, n = 165; Alzheimer's Disease Neuroimaging Initiative, n = 53) and who had Alzheimer's disease neuropathological change, limbic-predominant age-related TDP-43 encephalopathy or both pathologies at autopsy. These neuropathology-defined groups accounted for 35, 37 and 4% of cases in the Mayo cohort, respectively, and 30, 22 and 9% of cases in the Alzheimer's Disease Neuroimaging Initiative cohort, respectively. The criteria effectively categorized these cases, with Alzheimer's disease having the lowest likelihoods, limbic-predominant age-related TDP-43 encephalopathy patients having the highest likelihoods and patients with both pathologies having intermediate likelihoods. A logistic regression using the criteria features as predictors of TDP-43 achieved a balanced accuracy of 74.6% in the Mayo cohort, and out-of-sample predictions in an external cohort achieved a balanced accuracy of 73.3%. Patients with high likelihoods had a milder and slower clinical course and more severe temporo-limbic degeneration compared to those with low likelihoods. Stratifying patients with both Alzheimer's disease neuropathological change and limbic-predominant age-related TDP-43 encephalopathy from the Mayo cohort according to their likelihoods revealed that those with higher likelihoods had more temporo-limbic degeneration and a slower rate of decline and those with lower likelihoods had more lateral temporo-parietal degeneration and a faster rate of decline. The implementation of criteria for a limbic-predominant amnestic neurodegenerative syndrome has implications to disambiguate the different aetiologies of progressive amnestic presentations in older age and guide diagnosis, prognosis, treatment and clinical trials.
Keywords: Alzheimer’s disease; amnestic syndrome; behavioural neurology; limbic age-related 43 encephalopathy; limbic-predominant amnestic neurodegenerative syndrome.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
V.J.L. consults for Bayer Schering Pharma, Piramal Life Sciences, Life Molecular Imaging, Eisai Inc., Avid Radiopharmaceuticals and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, Avid Radiopharmaceuticals and the NIH (NIA, NCI). D.S.K. serves on a Data Safety Monitoring Board for the DIAN study and for a tau therapeutic for Biogen but receives no personal compensation; is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California; has served as a consultant for Roche, Samus Therapeutics, Third Rock and Alzeca Biosciences but receives no personal compensation; and receives funding from the NIH. B.F.B. receives honorarium for SAB activities for the Tau Consortium, is an investigator in clinical trials sponsored by Alector, Biogen, Cognition Therapeutics, EIP Pharma and Transposon and receives funding from the NIH. C.R.J. has no commercial conflicts and receives research support from the NIH, the GHR Foundation and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. R.C.P. consults for Roche, Inc.; Merck, Inc.; Biogen, Inc.; Genentech, Inc.; Eisai, Inc.; and Nestle, Inc. but does not receive significant fees due to NIH limitations from the U24 AG057437 Co-PI role.
Figures

Update of
-
A limbic-predominant amnestic neurodegenerative syndrome associated with TDP-43 pathology.medRxiv [Preprint]. 2023 Nov 20:2023.11.19.23298314. doi: 10.1101/2023.11.19.23298314. medRxiv. 2023. Update in: Brain Commun. 2024 Jul 17;6(4):fcae183. doi: 10.1093/braincomms/fcae183. PMID: 38045300 Free PMC article. Updated. Preprint.
References
-
- Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: A common pathological feature of dementia in very old (≥80 years of age) humans. Acta Neuropathol. 1994;88(3):212–221. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
