SARS-CoV-2 anti-RBD and anti-N protein responses are differentially regulated between mother-child pairs: insight from a national study cohort at the Faroe Islands
- PMID: 39021574
- PMCID: PMC11251900
- DOI: 10.3389/fimmu.2024.1418678
SARS-CoV-2 anti-RBD and anti-N protein responses are differentially regulated between mother-child pairs: insight from a national study cohort at the Faroe Islands
Abstract
Background: Knowledge about SARS-CoV-2 antibody dynamics in neonates and direct comparisons with maternal antibody responses are not well established. This study aimed to characterize and directly compare the maternal and infant antibody response in a national birth cohort from the Faroe Islands.
Methods: The levels of immunoglobulins (Ig) targeting the receptor binding domain (RBD) of the spike protein and the nucleocapsid protein (N protein) of SARS-CoV-2 were investigated in maternal blood and umbilical cord blood from neonates. The study included 537 neonates and 565 mothers from the Faroe Islands, and follow-up samples were collected 12 months after birth. Multiple linear regression models were used to assess associations of maternal parameters with maternal and neonatal Ig levels and pregnancy outcomes.
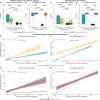
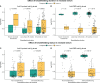
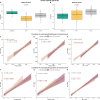
Results: The finding showed that neonates acquired varying levels of SARS-CoV-2 antibodies through transplacental transfer, and the levels were significantly influenced by the mother's vaccination and infection status. The study also found that maternal vaccination and the presence of SARS-CoV-2 antibodies targeting spike RBD were associated with gestational age and APGAR scores. Furthermore, the anti-RBD and -N protein-specific antibody response dynamics during 12 months after birth exhibited differences between mothers and children. RBD and N protein responses were maintained at follow-up in the mother's cohort, while only the N protein response was maintained at follow-up in the children's cohort.
Conclusion: In conclusion, SARS-CoV-2-specific immune responses in newborns rely on maternal immunity, while the persistence of SARS-CoV-2-specific Igs appears to be differently regulated between mothers and children. The study provides new insights into the dynamics of SARS-CoV-2-specific immune responses in newborns and underscores the nuanced relationship between maternal factors and neonatal humoral responses.
Keywords: SARS-CoV-2; antibody duration; maternal immunization; nucleocapsid protein; spike protein.
Copyright © 2024 Jarlhelt, Hansen, Pérez-Alós, Weihe, Petersen and Garred.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- World Health Organization. 2019 Coronavirus disease (COVID-19) pandemic. Geneva, Switzerland: World Health Organization. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed October 18, 2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

