Effect of FADS1 SNPs rs174546, rs174547 and rs174550 on blood fatty acid profiles and plasma free oxylipins
- PMID: 39021601
- PMCID: PMC11253720
- DOI: 10.3389/fnut.2024.1356986
Effect of FADS1 SNPs rs174546, rs174547 and rs174550 on blood fatty acid profiles and plasma free oxylipins
Abstract
Introduction: Previous studies have indicated that activity of fatty acid desaturase 1 (FADS1), is involved in cardiometabolic risk. Recent experimental data have shown that FADS1 knockdown can promote lipid accumulation and lipid droplet formation in liver cells. In this study, we aimed to characterize whether different FADS1 genotypes affect liver fat content, essential fatty acid content and free oxylipin mediators in the blood.
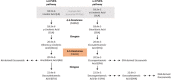
Methods: We analyzed the impact of FADS1 single-nucleotide polymorphisms (SNPs) rs174546, rs174547, and rs174550 on blood fatty acids and free oxylipins in a cohort of 85 patients from an academic metabolic medicine outpatient center. Patients were grouped based on their genotype into the homozygous major (derived) allele group, the heterozygous allele group, and the homozygous minor (ancestral) allele group. Omega-3 polyunsaturated fatty acids (n-3 PUFA) and omega-6 polyunsaturated fatty acids (n-6 PUFA) in the blood cell and plasma samples were analyzed by gas chromatography. Free Oxylipins in plasma samples were analyzed using HPLC-MS/MS. Liver fat content and fibrosis were evaluated using Fibroscan technology.
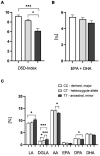
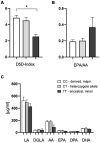
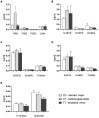
Results: Patients with the homozygous ancestral (minor) FADS1 genotype exhibited significantly lower blood levels of the n-6 PUFA arachidonic acid (AA), but no significant differences in the n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). There were no significant differences in liver fat content or arachidonic acid-derived lipid mediators, such as thromboxane B2 (TXB2), although there was a trend toward lower levels in the homozygous ancestral genotype group.
Discussion: Our findings suggest that FADS1 genotypes influence the blood levels of n-6 PUFAs, while not significantly affecting the n-3 PUFAs EPA and DHA. The lack of significant differences in liver fat content and arachidonic acid-derived lipid mediators suggests that the genotype-related variations in fatty acid levels may not directly translate to differences in liver fat or inflammatory lipid mediators in this cohort. However, the trend towards lower levels of certain lipid mediators in the homozygous ancestral genotype group warrants further investigation to elucidate the underlying mechanisms of different FADS1 genotypes and potential implications for cardiometabolic risk.
Keywords: FADS1; MAFLD; Oxylipins; PUFA; steatosis hepatis.
Copyright © 2024 Rabehl, Wei, Leineweber, Enssle, Rothe, Jung, Schmöcker, Elbelt, Weylandt and Pietzner.
Conflict of interest statement
MRo is owner of Lipidomix GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

