The Scr and Csc pathways for sucrose utilization co-exist in E. coli, but only the Scr pathway is widespread in other Enterobacteriaceae
- PMID: 39021635
- PMCID: PMC11251922
- DOI: 10.3389/fmicb.2024.1409295
The Scr and Csc pathways for sucrose utilization co-exist in E. coli, but only the Scr pathway is widespread in other Enterobacteriaceae
Abstract
Most Escherichia coli isolates from humans do not utilize D-sucrose as a substrate for fermentation or growth. Previous work has shown that the Csc pathway allows some E. coli to utilize sucrose for slow growth, and this pathway has been engineered in E. coli W strains to enhance use of sucrose as a feedstock for industrial applications. An alternative sucrose utilization pathway, Scr, was first identified in Klebsiella pneumoniae and has been reported in some E. coli and Salmonella enterica isolates. We show here that the Scr pathway is native to an important subset of E. coli phylogroup B2 lineages that lack the Csc pathway but grow rapidly on sucrose. Laboratory E. coli strains derived from MG1655 (phylogroup A, ST10) are unable to utilize sucrose and lack the scr and csc genes, but a recombinant plasmid-borne scr locus enables rapid growth on and fermentation of sucrose. Genome analyses of Enterobacteriaceae indicate that the scr locus is widespread in other Enterobacteriaceae; including Enterobacter and Klebsiella species, and some Citrobacter and Proteus species. In contrast, the Csc pathway is limited mostly to E. coli, some Shigella species (in which csc loci are rendered non-functional by various mutations), and Citrobacter freundii. The more efficient Scr pathway likely has greater potential than the Csc pathway for bioindustrial applications of E. coli and other Enterobacteriaceae using sucrose as a feedstock.
Keywords: Csc; Escherichia coli; Scr; evolutionary genomics; metabolism; sucrose.
Copyright © 2024 Stephens, Martinez, Leonardi, Jaing and Miller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
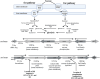
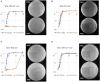
Figures





References
-
- Alaeddinoglu N. G., Charles H. P. (1979). Transfer of a gene for sucrose utilization into Escherichia coli K12, and consequent failure of expression of genes for D-serine utilization. Microbiology 110, 47–59. - PubMed
LinkOut - more resources
Full Text Sources

