Are Orthopaedic Clinical Trials Linguistically and Culturally Diverse? A Systematic Review
- PMID: 39021638
- PMCID: PMC11250675
- DOI: 10.2106/JBJS.RVW.24.00012
Are Orthopaedic Clinical Trials Linguistically and Culturally Diverse? A Systematic Review
Abstract
Purpose: Underrepresentation and misrepresentation of historically underrepresented populations in randomized controlled trials (RCTs) may have implications for the validity of research results and their application for diverse populations. To evaluate the representation of historically linguistically, racially, and ethnically underrepresented participants in orthopaedic randomized controlled trials (RCTs) and to assess the use of translated and culturally adapted patient reported outcome measures (PROMs).
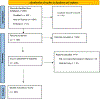
Methods: Separate and comprehensive literature searches of PubMed, Web of Science, and Embase databases were performed to identify RCTs utilizing PROMs between the years 2012 - 2022 among the top five highest 5-year impact factor orthopaedic journals according to the 2021 Journal Citation Reports database. The primary outcomes of interest included reporting of linguistic, racial and ethnic demographic characteristics of trial participants and the utilization of translated PROMs. The methodological quality of each clinical trial was assessed using the Jadad Criteria.
Results: 230 RCTs met inclusion criteria. The language of participants was reported in 14% of trials and in 17% of trials when searching both the published text and clinical trial registration information. In addition, race and/or ethnicity was reported in 11% of trials, and the use of translated PROMs was reported in 7% of trials. Among the six multinational studies, none reported the language of the study population nor the use of translated PROMs. Notably, four studies (2%) reported utilizing culturally adapted PROMs. The average Jadad score was 3.07.
Conclusion: Participant language, race, and ethnicity are infrequently reported in orthopaedic clinical trials, potentially limiting the application and interpretation of study results. Similarly, the linguistic and cultural adaptation of PROMs utilized are often not reported, which also limits interpretations of the validity and generalizability of orthopedic study results. Researchers and journals should promote standard reporting of demographic data and methods of PROM adaptation to ensure results are generalizable to diverse patient populations.
Level of evidence: III.
Keywords: Cultural Adaptation; Diversity; Equity; Inclusion; Randomized Controlled Trials; Representation; Translation.
Conflict of interest statement
Competing Interests Statement: The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Hays RD, Calderón JL, Spritzer KL, Reise SP, & Paz SH (2018). Differential item functioning by language on the PROMIS® physical functioning items for children and adolescents. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation, 27(1), 235–247 - PMC - PubMed
-
- Walsh M, Davidovitch RI, & Egol KA (2010). Ethnic disparities in recovery following distal radial fracture. The Journal of bone and joint surgery. American volume, 92(5), 1082–1087 - PubMed
-
- Paz SH, Spritzer KL, Morales LS, & Hays RD (2013). Evaluation of the Patient-Reported Outcomes Information System (PROMIS(®)) Spanish-language physical functioning items. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation, 22(7), 1819–1830 - PMC - PubMed
-
- Hao KA, Kakalecik J, Delgado GA, Wright TW, King JJ, Wright JO, et al.: Current trends in patient-reported outcome measures for clavicle fractures: a focused systematic review of 11 influential orthopaedic journals. J Shoulder Elbow Surg 2022;31(2):e58–e67 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

