Metabolic bottlenecks of Pseudomonas taiwanensis VLB120 during growth on d-xylose via the Weimberg pathway
- PMID: 39021639
- PMCID: PMC11252243
- DOI: 10.1016/j.mec.2024.e00241
Metabolic bottlenecks of Pseudomonas taiwanensis VLB120 during growth on d-xylose via the Weimberg pathway
Abstract
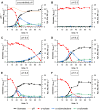
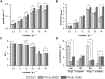
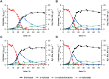
The microbial production of value-added chemicals from renewable feedstocks is an important step towards a sustainable, bio-based economy. Therefore, microbes need to efficiently utilize lignocellulosic biomass and its dominant constituents, such as d-xylose. Pseudomonas taiwanensis VLB120 assimilates d-xylose via the five-step Weimberg pathway. However, the knowledge about the metabolic constraints of the Weimberg pathway, i.e., its regulation, dynamics, and metabolite fluxes, is limited, which hampers the optimization and implementation of this pathway for bioprocesses. We characterized the Weimberg pathway activity of P. taiwanensis VLB120 in terms of biomass growth and the dynamics of pathway intermediates. In batch cultivations, we found excessive accumulation of the intermediates d-xylonolactone and d-xylonate, indicating bottlenecks in d-xylonolactone hydrolysis and d-xylonate uptake. Moreover, the intermediate accumulation was highly dependent on the concentration of d-xylose and the extracellular pH. To encounter the apparent bottlenecks, we identified and overexpressed two genes coding for putative endogenous xylonolactonases PVLB_05820 and PVLB_12345. Compared to the control strain, the overexpression of PVLB_12345 resulted in an increased growth rate and biomass generation of up to 30 % and 100 %, respectively. Next, d-xylonate accumulation was decreased by overexpressing two newly identified d-xylonate transporter genes, PVLB_18545 and gntP (PVLB_13665). Finally, we combined xylonolactonase overexpression with enhanced uptake of d-xylonate by knocking out the gntP repressor gene gntR (PVLB_13655) and increased the growth rate and biomass yield by 50 % and 24 % in stirred-tank bioreactors, respectively. Our study contributes to the fundamental knowledge of the Weimberg pathway in pseudomonads and demonstrates how to encounter the metabolic bottlenecks of the Weimberg pathway to advance strain developments and cell factory design for bioprocesses on renewable feedstocks.
Keywords: Pseudomonas taiwanensis VLB120; Renewable feedstocks; Weimberg pathway; Xylonate transport; Xylonolactonase; Xylose utilization.
© 2024 The Authors. Published by Elsevier B.V. on behalf of International Metabolic Engineering Society.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
D-Xylose assimilation via the Weimberg pathway by solvent-tolerant Pseudomonas taiwanensis VLB120.Environ Microbiol. 2015 Jan;17(1):156-70. doi: 10.1111/1462-2920.12537. Epub 2014 Jul 15. Environ Microbiol. 2015. PMID: 24934825
-
Identification of modifications procuring growth on xylose in recombinant Saccharomyces cerevisiae strains carrying the Weimberg pathway.Metab Eng. 2019 Sep;55:1-11. doi: 10.1016/j.ymben.2019.05.010. Epub 2019 May 28. Metab Eng. 2019. PMID: 31150803
-
Exploring D-xylose oxidation in Saccharomyces cerevisiae through the Weimberg pathway.AMB Express. 2018 Mar 5;8(1):33. doi: 10.1186/s13568-018-0564-9. AMB Express. 2018. PMID: 29508097 Free PMC article.
-
Microbial D-xylonate production.Appl Microbiol Biotechnol. 2012 Oct;96(1):1-8. doi: 10.1007/s00253-012-4288-5. Epub 2012 Aug 9. Appl Microbiol Biotechnol. 2012. PMID: 22875400 Free PMC article. Review.
-
Everyone loves an underdog: metabolic engineering of the xylose oxidative pathway in recombinant microorganisms.Appl Microbiol Biotechnol. 2018 Sep;102(18):7703-7716. doi: 10.1007/s00253-018-9186-z. Epub 2018 Jul 12. Appl Microbiol Biotechnol. 2018. PMID: 30003296 Review.
Cited by
-
Metabolic engineering for microbial production of sugar acids.BMC Biotechnol. 2025 May 13;25(1):36. doi: 10.1186/s12896-025-00973-7. BMC Biotechnol. 2025. PMID: 40361067 Free PMC article. Review.
-
Engineered Passive Glucose Uptake in Pseudomonas taiwanensis VLB120 Increases Resource Efficiency for Bioproduction.Microb Biotechnol. 2025 Jan;18(1):e70095. doi: 10.1111/1751-7915.70095. Microb Biotechnol. 2025. PMID: 39871105 Free PMC article.
References
-
- Bardhan S.K., Gupta S., Gorman M.E., Haider M.A. Biorenewable chemicals: feedstocks, technologies and the conflict with food production. Renew. Sustain. Energy Rev. 2015;51:506–520. doi: 10.1016/j.rser.2015.06.013. - DOI
-
- Bator I. Deep genome editing of Pseudomonas putida for rhamnolipid production using non-conventional substrates. Apprimus Verlag, Aachen. 2021 doi: 10.18154/RWTH-2021-00901. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

