TGF-β1 promotes osteogenesis of mesenchymal stem cells via integrin mediated mechanical positive autoregulation
- PMID: 39021801
- PMCID: PMC11253692
- DOI: 10.1016/j.isci.2024.110262
TGF-β1 promotes osteogenesis of mesenchymal stem cells via integrin mediated mechanical positive autoregulation
Abstract
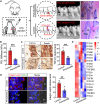
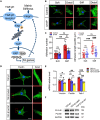
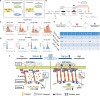
Positive autoregulation (PAR), one type of network motifs, provides a high phenotypic heterogeneity for cells to better adapt to their microenvironments. Typical mechanosensitive proteins can also form PAR, e.g., integrin mediated PAR, but the role of such mechanical PAR in physiological development and pathological process remains elusive. In this study, we found that transforming growth factor β1 (TGF-β1) and integrin levels decrease with tissue softening after the development of paradentium in vivo in rat model of periodontitis (an inflammatory disease with bone defect). Interestingly, TGF-β1 could induce the formation of mechanical PAR involving the integrin-FAK-YAP axis in mesenchymal stem cells (MSCs) by both in vitro experiments and in silico computational model. The computational model predicted a mechanical PAR involving the bimodal distribution of focus adhesions, which enables cells to accurately perceive extracellular mechanical cues. Thus, our analysis of TGF-β1 mediated mechanosensing mechanism on MSCs may help to better understand the molecular process underlying bone regeneration.
Keywords: Biological sciences; Cell biology; Mathematical biosciences; Mechanobiology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

