Hepatocellular Carcinoma Patients with Hepatitis B Virus Infection Exhibited Favorable Survival from Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis
- PMID: 39021889
- PMCID: PMC11250578
- DOI: 10.1159/000534446
Hepatocellular Carcinoma Patients with Hepatitis B Virus Infection Exhibited Favorable Survival from Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis
Abstract
Background: Immune checkpoint inhibitors (ICIs) have demonstrated effectiveness for advanced hepatocellular carcinoma (HCC). However, the discrepancy in the efficacy of ICIs in HCC patients with distinct etiologies has not been systematically validated.
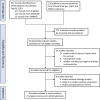
Methods: PubMed, MEDLINE, Embase, clinicaltrials.gov, and abstracts from ASCO and ESMO conferences were searched for eligible trials that explored the impact of etiology factor on the ICI treatment in HCC patients. The pooled hazard ratio (HR) of overall survival (OS) and progression-free survival (PFS), as well as the pooled odds ratio (OR) of objective response rate (ORR), were calculated with stratification of hepatitis B virus (HBV), hepatitis C virus (HCV) and nonviral subgroup, and the heterogeneity between different etiological subgroups was assessed by using an interaction test.
Results: Eight eligible studies with a total of 5,646 patients were identified from searching published articles and conference abstracts. ICI therapies were associated with significantly prolonged OS with the pooled HRs of 0.78 (95% CI 0.73-0.84, p < 0.001), 0.71 (95% CI 0.65-0.79, p < 0.001), 0.80 (95% CI 0.69-0.93, p = 0.003), and 0.87 (95% CI 0.77-0.97, p = 0.011) for the whole population, HBV subgroup, HCV subgroup, and non-viral subgroup, respectively. In addition, this analysis reported a significant PFS improvement with ICI therapies with HRs of 0.78 (p = 0.004), 0.53 (p < 0.001), 0.65 (p = 0.011), and 0.81 (p = 0.107) for whole population, HBV, HCV, and nonviral subgroup, respectively. The HBV-related HCC patients showed the more distinctive HRs for OS and PFS than other etiology subgroups, and this difference was significant in PFS (p for heterogeneity = 0.001), and there was a tendency of significance in OS (p for heterogeneity = 0.079). Furthermore, the ORR advantages of ICI therapies over control were also confirmed with the pooled ORs of 3.62 (p < 0.001), 3.84 (p < 0.001), 3.05 (p < 0.001), and 2.99 (p < 0.001) for whole population, HBV, HCV, and nonviral population, respectively (p for heterogeneity = 0.743).
Conclusions: ICI therapies significantly improve OS, PFS, and ORR for HCC patients with different etiologies. HBV-related HCC patients could be the highlighted population to benefit from ICI treatment.
Keywords: Etiology; Hepatitis B virus; Hepatocellular carcinoma; Immune checkpoint inhibitor; Meta-analysis.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Zhenggang Ren is serving in a consulting or advisory role for AstraZeneca, Merck Sharp and Dohme, and Roche and other authors declare no potential conflicts of interest.
Figures
References
-
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52. - PubMed
-
- Kudo M, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer DH, et al. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer. 2022;167:1–12. - PubMed
LinkOut - more resources
Full Text Sources