The development of an innovative method to improve the dissolution performance of rivaroxaban
- PMID: 39021978
- PMCID: PMC11253053
- DOI: 10.1016/j.heliyon.2024.e33162
The development of an innovative method to improve the dissolution performance of rivaroxaban
Abstract
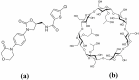
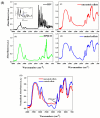
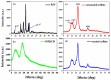
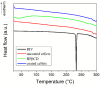
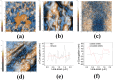
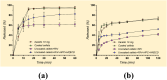
Recent advancements in the formulation of solid dosage forms involving active ingredient-cyclodextrin complexes have garnered considerable attention in pharmaceutical research. While previous studies predominantly focused on incorporating these complexes into solid states, issues regarding incomplete inclusion prompted the exploration of novel methods. In this study, we aimed to develop an innovative approach to integrate liquid-state drug-cyclodextrin inclusion complexes into solid dosage forms. Our investigation centered on rivaroxaban, a hydrophobic compound practically insoluble in water, included in hydroxypropyl-β-cyclodextrin at a 1:1 M ratio, and maintained in a liquid state. To enhance viscosity, hydroxypropyl-cellulose (2 % w/w) was introduced, and the resulting dispersion was sprayed onto the surface of cellulose pellets (CELLETS®780) using a Caleva Mini Coater. The process parameters were meticulously controlled, with atomization air pressure set at 1.1 atm and a fluidizing airflow maintained at 35-45 m3/h. Characterization of the coated cellets, alongside raw materials, was conducted using Fourier Transform Infrared Spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and differential scanning calorimetry (DSC) analyses. Physicochemical evaluations affirmed the successful incorporation of rivaroxaban into hydroxypropyl-β-cyclodextrin, with the final cellets demonstrating excellent flowability, compressibility, and adequate hardness. Quantitative analysis via the HPLC-DAD method confirmed a drug loading of 10 mg rivaroxaban/750 mg coated cellets. In vitro dissolution studies were performed in two distinct media: 0.022 M sodium acetate buffer pH 4.5 with 0.2 % sodium dodecyl sulfate (mirroring compendial conditions for 10 mg rivaroxaban tablets), and 0.05 M phosphate buffer pH 6.8 without surfactants, compared to reference capsules and conventional tablet formulations. The experimental capsules exhibited similar release profiles to the commercial product, Xarelto® 10 mg, with enhanced dissolution rates observed within the initial 10 min. This research presents a significant advancement in the development of solid dosage forms incorporating liquid-state drug-cyclodextrin inclusion complexes, offering a promising avenue for improving drug delivery and bioavailability.
Keywords: Dissolution rate; Drug release; Hydroxypropyl-β-cyclodextrin; Inclusion complexes; Rivaroxaban; Solid dosage forms.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Celebioglu A., Saporito A.F., Uyar T. Green electrospinning of chitosan/pectin nanofibrous films by the incorporation of cyclodextrin/curcumin inclusion complexes: pH-responsive release and hydrogel features. ACS Sustainable Chem. Eng. 2022;10(14):4758–4769. doi: 10.1021/acssuschemeng.2c00650. - DOI
-
- Yang G., Li F., Zhang H., Yan H., Gao S., Fu Y., Ye F. Electrospinning for producing antifungal nanofibers consisting of prochloraz/hydroxypropyl-γ-cyclodextrin inclusion complex. Ind. Crop. Prod. 2024;211 doi: 10.1016/j.indcrop.2024.118282. - DOI
-
- Zhang Y., Li F., Guo G., Xiu Y., Yan H., Zhao L., Gao S., Ye F., Fu Y. Preparation and characterization of betulin/methyl-beta-cyclodextrin inclusion complex electrospun nanofiber: improving the properties of betulin. Ind. Crop. Prod. 2024;209 doi: 10.1016/j.indcrop.2023.117974. - DOI
-
- Liu B., Duan J., Zhang Y., Wang R., Zhao L., Gao S., Ye F., Fu Y. Preparation and characterization of hexaconazole/hydroxypropyl-gamma-cyclodextrin inclusion complex nanofibers for sustainable agriculture: improved physicochemical properties and antifungal activity of hexaconazole. J. Mol. Struct. 2024;1299 doi: 10.1016/j.molstruc.2023.137195. - DOI
-
- Feng W., Guo X., Yang G., Yao Y., Zhao L., Gao S., Ye F., Fu Y. Direct electrospinning for producing multiple activity nanofibers consisting of aggregated luteolin/hydroxypropyl-gamma-cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2024;270(Part 1) doi: 10.1016/j.ijbiomac.2024.132344. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

