Neighbor effect on conformational spaces of alanine residue in azapeptides
- PMID: 39021983
- PMCID: PMC11253059
- DOI: 10.1016/j.heliyon.2024.e33159
Neighbor effect on conformational spaces of alanine residue in azapeptides
Abstract
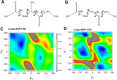
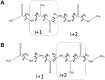
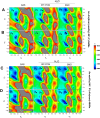
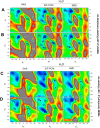
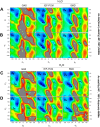
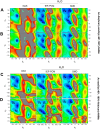
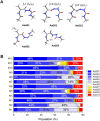
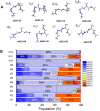
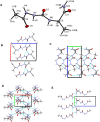
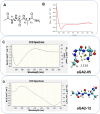
The conformational properties of Alanine (Ala) residue have been investigated to understand protein folding and develop force fields. In this work, we examined the neighbor effect on the conformational spaces of Ala residue using model azapeptides, Ac-Ala-azaGly-NHMe (3, AaG), and Ac-azaGly-Ala-NHMe (4, aGA1). Ramachandran energy maps were generated by scanning (φ, ψ) dihedral angles of the Ala residues in models with the fixed dihedral angles (φ = ±90°, ψ = ±0° or ±180°) of azaGly residue using LCgau-BOP and LCgau-BOP + LRD functionals in the gas and water phases. The integral-equation-formalism polarizable continuum model (IEF-PCM) and a solvation model density (SMD) were employed to mimic the solvation effect. The most favorable conformation of Ala residue in azapeptide models is found as the polyproline II (βP), inverse γ-turn (γ'), β-sheet (βS), right-handed helix (αR), or left-handed helix (αL) depending on the conformation of neighbor azaGly residue in isolated form. Solvation methods exhibit that the Ala residue favors the βP, δR, and αR conformations regardless of its position in azapeptides 3 and 4 in water. Azapeptide 5, Ac-azaGly-Ala-NH2 (aGA2), was synthesized to evaluate the theoretical results. The X-ray structure showed that azaGly residue adopts the polyproline II (βP) and Ala residue adopts the right-handed helical (αR) structure in aGA2. The conformational preferences of aGA2 and the dimer structure of aGA2 based on the X-ray structure were examined to assess the performance of DFT functionals. In addition, the local minima of azapeptide 6, Ac-Phe-azaGly-NH2 (FaG), were compared with the previous experimental results. SMD/LCgau-BOP + LRD methods agreed well with the reported experimental results. The results suggest the importance of weak dispersion interactions, neighbor effect, and solvent influence in the conformational preferences of Ala residue in model azapeptides.
Keywords: And Lcgau-BOP+LRD; Azapeptide; DFT functionals; Foldamer; LCgau-BOP; βI-turn; βII-turn.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A theoretical study of conformational properties of N-methyl azapeptide derivatives.J Am Chem Soc. 2002 Oct 9;124(40):11881-93. doi: 10.1021/ja026496x. J Am Chem Soc. 2002. PMID: 12358532
-
Computational Investigation of Conformational Properties of Short Azapeptides: Insights from DFT Study and NBO Analysis.Molecules. 2023 Jul 17;28(14):5454. doi: 10.3390/molecules28145454. Molecules. 2023. PMID: 37513326 Free PMC article.
-
The beta-turn scaffold of tripeptide containing an azaphenylalanine residue.Biophys Chem. 2007 Jan;125(1):117-26. doi: 10.1016/j.bpc.2006.05.028. Epub 2006 Aug 4. Biophys Chem. 2007. PMID: 16890344
-
Designing of peptides with left handed helical structure by incorporating the unusual amino acids.Indian J Biochem Biophys. 1999 Jun;36(3):195-203. Indian J Biochem Biophys. 1999. PMID: 10650718
-
Azapeptides and their therapeutic potential.Future Med Chem. 2011 Jul;3(9):1139-64. doi: 10.4155/fmc.11.74. Future Med Chem. 2011. PMID: 21806378 Review.
References
-
- Perczel A., Angyan J.G., Kajtar M., Viviani W., Rivail J.L., Marcoccia J.F., Csizmadia I.G. Peptide models. 1. Topology of selected peptide conformational potential energy surfaces (glycine and alanine derivatives) J. Am. Chem. Soc. 1991;113(16):6256–6265.
-
- Head-Gordon T., Head-Gordon M., Frisch M.J., Brooks C.L., III Pople JA: theoretical study of blocked glycine and alanine peptide analogs. J. Am. Chem. Soc. 1991;113(16):5989–5997.
-
- Perczel A., Farkas O., Jákli I., Topol I.A., Csizmadia I.G. Peptide models XXXIII. Extrapolation of low-level Hartree-Fock data of peptide conformation to large basis set SCF, MP2, DFT, and CCSD(T) results. The Ramachandran surface of alanine dipeptide computed at various levels of theory. J. Comput. Chem. 2003;24(9):1026–1042. - PubMed
-
- Hudáky I., Hudáky P., Perczel A. Solvation model induced structural changes in peptides. A quantum chemical study on Ramachandran surfaces and conformers of alanine diamide using the polarizable continuum model. J. Comput. Chem. 2004;25(12):1522–1531. - PubMed
-
- Wang Z.X., Duan Y. Solvation effects on alanine dipeptide: a MP2/cc-pVTZ//MP2/6-31G** study of (Phi, Psi) energy maps and conformers in the gas phase, ether, and water. J. Comput. Chem. 2004;25(14):1699–1716. - PubMed
Associated data
LinkOut - more resources
Full Text Sources

