Synovial fibroblasts from children with oligoarticular juvenile idiopathic arthritis induce migration and prolong viability of neutrophils
- PMID: 39022217
- PMCID: PMC11251878
- DOI: 10.3389/fped.2024.1376371
Synovial fibroblasts from children with oligoarticular juvenile idiopathic arthritis induce migration and prolong viability of neutrophils
Abstract
Introduction: Little is known of the processes that trigger neutrophil activation in the joint of patients with oligoarticular juvenile idiopathic arthritis (oJIA), and if synovial fibroblasts (S-Fib) play an important role in the activation. Therefore, we aimed to investigate whether S-Fib derived from oJIA patients drive neutrophil activation.
Methods: Synovial fluid (SF) was collected from patients with oJIA. S-Fib were isolated from the SF of n = 7 patients through passaging. Subsequently, the S-Fib were primed or not with 20% of pooled SF. Supernatants were used to study migration of neutrophils in a transwell system. Additionally, the influence of S-Fib on neutrophils were studied in co-cultures. Phenotype and viability were assessed by flow cytometry. Neutrophil function was tested through the production of reactive oxygen species (ROS), and supernatants were tested for myeloperoxidase (MPO) release and elastase activity.
Results: Supernatants of S-Fib induced neutrophil migration (n = 5, p = 0.0491), which was further pronounced using supernatants from SF-primed S-Fib (p = 0.0063). Additionally, co-culture between SF-primed S-Fib and neutrophils resulted in prolonged viability (n = 5, p = 0.0094), with little effect on activation markers, e.g., CD11b. Conversely, co-culture did not induce functional alterations (n = 4), such as production of ROS (p > 0.1570), release of MPO (p > 0.4934) or elastase activity (p > 0.0904). Finally, supernatant stimulation did not replicate the results of prolonged viability (p = 0.9102), suggesting a role of cell-contact.
Conclusion: S-Fib from patients with oJIA induce migration of neutrophils via soluble mediators and, in addition, S-Fib prolong neutrophil viability in a cell-contact dependent manner. These mechanisms could be important for accumulation of neutrophils during arthritis.
Keywords: fibroblasts; inflammation; juvenile idiopathic arthritis; neutrophils; rheumatology.
© 2024 Schmidt, Mossberg, Berthold, Król, Linge, Bengtsson, Kahn, Månsson and Kahn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

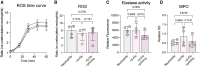
References
-
- Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. (2019) 46(2):190–7. 10.3899/jrheum.180168 - DOI - PubMed
-
- Arve-Butler S, Schmidt T, Mossberg A, Berthold E, Gullstrand B, Bengtsson AA, et al. Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res Ther. (2021) 23(1):109. 10.1186/s13075-021-02483-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

