Changes in neuroinflammatory biomarkers correlate with disease severity and neuroimaging alterations in patients with COVID-19 neurological complications
- PMID: 39022627
- PMCID: PMC11253226
- DOI: 10.1016/j.bbih.2024.100805
Changes in neuroinflammatory biomarkers correlate with disease severity and neuroimaging alterations in patients with COVID-19 neurological complications
Abstract
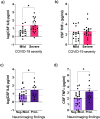
COVID-19 induces acute and persistent neurological symptoms in mild and severe cases. Proposed concomitant mechanisms include direct viral infection and strain, coagulopathy, hypoxia, and neuroinflammation. However, underlying molecular alterations associated with multiple neurological outcomes in both mild and severe cases are majorly unexplored. To illuminate possible mechanisms leading to COVID-19 neurological disease, we retrospectively investigated in detail a cohort of 35 COVID-19 mild and severe hospitalized patients presenting neurological alterations subject to clinically indicated cerebrospinal fluid (CSF) sampling. Clinical and neurological investigation, brain imaging, viral sequencing, and cerebrospinal CSF analyses were carried out. We found that COVID-19 patients presented heterogeneous neurological symptoms dissociated from lung burden. Nasal swab viral sequencing revealed a dominant strain at the time of the study, and we could not detect traces of SARS-CoV-2's spike protein in patients' CSF by multiple reaction monitoring analysis. Patients presented ubiquitous systemic hyper-inflammation and broad alterations in CSF proteomics related to inflammation, innate immunity, and hemostasis, irrespective of COVID-19 severity or neuroimaging alterations. Elevated CSF interleukin-6 (IL6) correlated with disease severity (sex-, age-, and comorbidity-adjusted mean Severe 24.5 pg/ml, 95% confidence interval (CI) 9.62-62.23 vs. Mild 3.91 pg/mL CI 1.5-10.3 patients, p = 0.019). CSF tumor necrosis factor-alpha (TNFα) and IL6 levels were higher in patients presenting pronounced neuroimaging alterations compared to those who did not (sex-, age-, and comorbidity-adjusted mean TNFα Pronounced 3.4, CI 2.4-4.4 vs. Non-Pronounced 2.0, CI 1.4-2.5, p = 0.022; IL6 Pronounced 33.11, CI 8.89-123.31 vs Non-Pronounced 6.22, CI 2.9-13.34, p = 0.046). Collectively, our findings put neuroinflammation as a possible driver of COVID-19 acute neurological disease in mild and severe cases.
Keywords: COVID-19; Coronavirus; Inflammation; Neuro-infectious diseases; Neuroinflammation.
© 2024 The Authors.
Conflict of interest statement
none.
Figures




References
-
- Alvim R.G., Lima T.M., Rodrigues D.A.S., Marsili F.F., Bozza V.B.T., Higa L.M., Monteiro F.L., Leitão I.C., Carvalho R.S., Galliez R.M., Castineiras T.M.P.P., Nobrega A., Travassos L.H., Ferreira Jr OC., Tanuri A., Vale A.M., Castilho L.R. An affordable anti-SARS-COV-2 spike protein ELISA test for early detection of IgG seroconversion suited for large-scale surveillance studies in low-income countries. medRxiv. 2020 doi: 10.1101/2020.07.13.20152884. - DOI
-
- Angriman F., Ferreyo B., Burry L., Fan E. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir. Med. 2021;9:655–664. https://pubmed.ncbi.nlm.nih.gov/33930329/ Available at: - PMC - PubMed
-
- Candido D.S., et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369(1979):1255–1260. https://pubmed.ncbi.nlm.nih.gov/32703910/ Available at: - PMC - PubMed
-
- Chou S.H.-Y., Beghi E., Helbok R., Moro E., Sampson J., Altamirano V., Mainali S., Bassetti C., Suarez J.I., McNett M., GCS-NeuroCOVID Consortium and ENERGY Consortium Global Incidence of neurological manifestations among patients hospitalized with COVID-19-A report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Neurol. 2021;4 http://www.ncbi.nlm.nih.gov/pubmed/33974053 Available at: - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

