Pathways to hypermutation in high-grade gliomas: Mechanisms, syndromes, and opportunities for immunotherapy
- PMID: 39022645
- PMCID: PMC11252568
- DOI: 10.1093/noajnl/vdae105
Pathways to hypermutation in high-grade gliomas: Mechanisms, syndromes, and opportunities for immunotherapy
Abstract
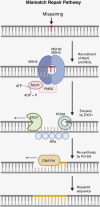
Despite rapid advances in the field of immunotherapy, including the success of immune checkpoint inhibition in treating multiple cancer types, clinical response in high-grade gliomas (HGGs) has been disappointing. This has been in part attributed to the low tumor mutational burden (TMB) of the majority of HGGs. Hypermutation is a recently characterized glioma signature that occurs in a small subset of cases, which may open an avenue to immunotherapy. The substantially elevated TMB of these tumors most commonly results from alterations in the DNA mismatch repair pathway in the setting of extensive exposure to temozolomide or, less frequently, from inherited cancer predisposition syndromes. In this review, we discuss the genetics and etiology of hypermutation in HGGs, with an emphasis on the resulting genomic signatures, and the state and future directions of immuno-oncology research in these patient populations.
Keywords: DNA mismatch repair; glioma; hypermutation; immune checkpoint inhibitor (ICI); temozolomide.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology and the European Association of Neuro-Oncology 2024.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
