Targeting genomic receptors in voided urine for confirmation of benign prostatic hyperplasia
- PMID: 39022663
- PMCID: PMC11250152
- DOI: 10.1002/bco2.362
Targeting genomic receptors in voided urine for confirmation of benign prostatic hyperplasia
Erratum in
-
Erratum.BJUI Compass. 2024 Dec 30;5(12):1324-1329. doi: 10.1002/bco2.482. eCollection 2024 Dec. BJUI Compass. 2024. PMID: 39744071 Free PMC article.
Abstract
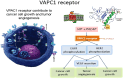
Objectives: The objective of this study is to validate a hypothesis that a non-invasive optical imaging assay targeting genomic VPAC receptors on malignant cells shed in voided urine will represent either benign prostatic hyperplasia (BPH) or prostatic cancer (PCa). Risk for BPH in men 50-70 years old is 50-70% and PCa is 17%. BPH and PCa can coexist in 20% of men with BPH. Most commonly practiced methods to diagnose BPH do not distinguish BPH from PCa.
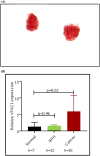
Patients or materials and methods: Males with BPH (N = 97, 60.8 ± 6.3 years, prostate-specific antigen 0.7 ± 0.4 ng/mL) and without oncologic disease (N = 35, 63.4 ± 5.8 years, prostate-specific antigen < 1.5 ng/mL) signed informed consent form and provided voided urine. Urine was cytocentrifuged, cells collected on glass slide, fixed, treated with VPAC specific fluorophore TP4303 (Kd 3.1 × 10-8M), washed, incubated with DAPI and observed using a fluorescence microscope. Cells with no VPAC did not fluoresce (BPH) and those with VPAC had red-orange fluorescence (PCa). Real-time polymerase chain reaction analyses for VPAC and NKX3.1 assay for cell origin were performed.
Results: Eighty-seven subjects were negative for VPAC expression. Positive VPAC expression was noted in 10 subjects. Patient chart review for clinical data on these 10 VPAC positive subjects showed five had nephrolithiasis, three had renal cysts, one had prostatitis and one was being treated with finasteride. Real-time polymerase chain reaction analysis-VPAC expressions for 7 normal and 12 BPH subjects were 1.31 ± 1.26 and 0.94 ± 0.89, respectively (P = 0.46). NKX3.1 showed cells of prostate origin for finasteride-treated patient. Specificity for VPAC urine assay for excluding prostate cancer in this BPH cohort was 88.5%, positive predictive value 0.00% and negative predictive value 100%.
Conclusion: VPAC assay may contribute extensively for BPH diagnosis and warrant continued investigation.
Keywords: BPH diagnosis; VPAC receptor assay; liquid biopsy and non‐invasive BPH diagnosis; voided urine assay.
© 2024 The Authors. BJUI Compass published by John Wiley & Sons Ltd on behalf of BJU International Company.
Conflict of interest statement
Drs. Mathew Thakur and Leonard Gomella hold patents on the use of TP4303 Biomolecule stated in this manuscript. Other authors declare that they have no known competing financial interests. None have personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122–S128. - PubMed
-
- Sandhu JS, Bixler BR, Dahm P, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia (BPH): AUA Guideline Amendment 2023. J Urol. 2023;211:1–8. - PubMed
-
- Uhr A, Glick L, Gomella LG. An overview of biomarkers in the diagnosis and management of prostate cancer. Can J Urol. 2020;27(S3):24–27. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
