The complement system in neurodegenerative and inflammatory diseases of the central nervous system
- PMID: 39022733
- PMCID: PMC11252048
- DOI: 10.3389/fneur.2024.1396520
The complement system in neurodegenerative and inflammatory diseases of the central nervous system
Abstract
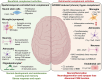
Neurodegenerative and neuroinflammatory diseases, including Alzheimer's disease, Parkinson's disease, and multiple sclerosis, affect millions of people globally. As aging is a major risk factor for neurodegenerative diseases, the continuous increase in the elderly population across Western societies is also associated with a rising prevalence of these debilitating conditions. The complement system, a crucial component of the innate immune response, has gained increasing attention for its multifaceted involvement in the normal development of the central nervous system (CNS) and the brain but also as a pathogenic driver in several neuroinflammatory disease states. Although complement is generally understood as a liver-derived and blood or interstitial fluid operative system protecting against bloodborne pathogens or threats, recent research, particularly on the role of complement in the healthy and diseased CNS, has demonstrated the importance of locally produced and activated complement components. Here, we provide a succinct overview over the known beneficial and pathological roles of complement in the CNS with focus on local sources of complement, including a discussion on the potential importance of the recently discovered intracellularly active complement system for CNS biology and on infection-triggered neurodegeneration.
Keywords: brain; central nervous system; complement; complosome; development; neurodegeneration; neuroinflammation.
Copyright © 2024 Negro-Demontel, Maleki, Reich and Kemper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

