Myelin basic protein mRNA levels affect myelin sheath dimensions, architecture, plasticity, and density of resident glial cells
- PMID: 39023138
- PMCID: PMC11426340
- DOI: 10.1002/glia.24589
Myelin basic protein mRNA levels affect myelin sheath dimensions, architecture, plasticity, and density of resident glial cells
Abstract
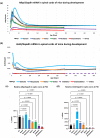
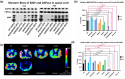
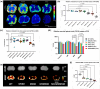
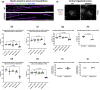
Myelin Basic Protein (MBP) is essential for both elaboration and maintenance of CNS myelin, and its reduced accumulation results in hypomyelination. How different Mbp mRNA levels affect myelin dimensions across the lifespan and how resident glial cells may respond to such changes are unknown. Here, to investigate these questions, we used enhancer-edited mouse lines that accumulate Mbp mRNA levels ranging from 8% to 160% of wild type. In young mice, reduced Mbp mRNA levels resulted in corresponding decreases in Mbp protein accumulation and myelin sheath thickness, confirming the previously demonstrated rate-limiting role of Mbp transcription in the control of initial myelin synthesis. However, despite maintaining lower line specific Mbp mRNA levels into old age, both MBP protein levels and myelin thickness improved or fully normalized at rates defined by the relative Mbp mRNA level. Sheath length, in contrast, was affected only when mRNA levels were very low, demonstrating that sheath thickness and length are not equally coupled to Mbp mRNA level. Striking abnormalities in sheath structure also emerged with reduced mRNA levels. Unexpectedly, an increase in the density of all glial cell types arose in response to reduced Mbp mRNA levels. This investigation extends understanding of the role MBP plays in myelin sheath elaboration, architecture, and plasticity across the mouse lifespan and illuminates a novel axis of glial cell crosstalk.
Keywords: Mbp transcription; astrocyte; hypermyelination; hypomyelination; microglia; myelin basic protein; myelin elaboration and plasticity; myelin sheath thickness and length; oligodendrocyte and oligodendrocyte progenitor cell.
© 2024 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

