Effect of L-arginine on cystic fibrosis Pseudomonas aeruginosa biofilms
- PMID: 39023260
- PMCID: PMC11304719
- DOI: 10.1128/aac.00336-24
Effect of L-arginine on cystic fibrosis Pseudomonas aeruginosa biofilms
Abstract
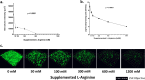
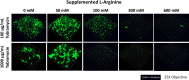
Cystic fibrosis (CF) airways are L-arginine deficient which may affect susceptibility to infection with certain pathogens. The potential impact of L-arginine supplementation on Pseudomonas aeruginosa, a common CF airway pathogen, is unclear. This study investigated the effects of L-arginine on P. aeruginosa biofilm cultures, using the laboratory strain PAO1 and multi-drug resistant CF clinical isolates. P. aeruginosa biofilms were grown in a chambered cover-glass slide model for 24 h, then exposed to either L-arginine alone or combined with tobramycin for an additional 24 h. Biofilms were visualized using confocal microscopy, and viable cells were measured via plating for colony-forming units. Increasing concentrations of L-arginine in bacterial culture medium reduced the biovolume of P. aeruginosa in a dose-dependent manner. Notably, L-arginine concentrations within the physiological range (50 mM and 100 mM) in combination with tobramycin promoted biofilm growth, while higher concentrations (600 mM and 1200 mM) inhibited growth. These findings demonstrate the potential of L-arginine as an adjuvant therapy to inhaled tobramycin in treating P. aeruginosa infections in people with CF.
Keywords: L-arginine; Pseudomonas aeruginosa; biofilm; cystic fibrosis; tobramycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

