Impact of hydroxyurea on follicle density in patients with sickle cell disease
- PMID: 39023361
- PMCID: PMC11530394
- DOI: 10.1182/bloodadvances.2023011536
Impact of hydroxyurea on follicle density in patients with sickle cell disease
Abstract
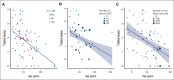
The impact of hydroxyurea (HU) on the ovarian reserve of female patients with sickle cell disease (SCD) remains poorly elucidated. Only direct histological analysis of ovarian follicle density can effectively evaluate HU's effect on ovarian reserve. By analyzing digitized slides of ovarian tissue from girls and young women with SCD who underwent ovarian tissue cryopreservation (OTC) before hematological stem cell transplantation, we meticulously counted follicles and categorized them based on their growth stage. We then calculated the densities of different follicle types and assessed their correlation with patient characteristics, clinical manifestations, and treatments extracted from medical records. Seventy-six patients with SCD participated in the study, with a median age at OTC of 10.2 years (interquartile range [IQR], 7.5-14.6), and 50 (65.8%) were prepubertal. Of these, 35 patients (46.1%) had received HU, with a median daily dosage of 23.0 mg/kg (IQR, 20.0-25.0) and median exposure time of 44 months (IQR, 24.0-54.0). Primordial follicle density was comparable between the HU and non-HU groups (5.8 follicles per mm2 [IQR, 1.0-13.3] vs 4.2 follicles per mm2 [IQR, 1.1-14.4], respectively; P = .95). However, in the HU group, after adjusting for age, the density of growing follicles was marginally lower than that in the non-HU group (P = .09). Notably, other parameters such as vaso-occlusive crisis did not affect follicular density. In conclusion, exposure to HU did not demonstrate a reduction in ovarian reserve in girls or women with SCD. Therefore, fertility preservation measures before initiating HU treatment do not seem necessary.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Comment in
-
Hydroxyurea and follicle density in sickle cell.Blood Adv. 2024 Oct 8;8(19):5225-5226. doi: 10.1182/bloodadvances.2024013815. Blood Adv. 2024. PMID: 39378027 Free PMC article. No abstract available.
References
-
- Therrell BL, Jr., Lloyd-Puryear MA, Eckman JR, Mann MY. Newborn screening for sickle cell diseases in the United States: a review of data spanning 2 decades. Semin Perinatol. 2015;39(3):238–251. - PubMed
-
- Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122(6):1332–1342. - PubMed
-
- Khera KS. A teratogenicity study on hydroxyurea and diphenylhydantoin in cats. Teratology. 1979;20(3):447–452. - PubMed
-
- Asano Y, Okaniwa A. In utero morphological effects of hydroxyurea on the fetal development in Sprague-Dawley rats. Jikken Dobutsu. 1987;36(2):143–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

