Ritlecitinib, a JAK3/TEC family kinase inhibitor, stabilizes active lesions and repigments stable lesions in vitiligo
- PMID: 39023568
- PMCID: PMC11258076
- DOI: 10.1007/s00403-024-03182-y
Ritlecitinib, a JAK3/TEC family kinase inhibitor, stabilizes active lesions and repigments stable lesions in vitiligo
Abstract
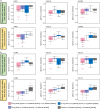
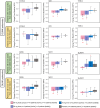
The efficacy of ritlecitinib, an oral JAK3/TEC family kinase inhibitor, on active and stable lesions was evaluated in patients with active non-segmental vitiligo in a phase 2b trial (NCT03715829). Patients were randomized to placebo or daily ritlecitinib 50 mg (with or without 4-week 100-mg or 200-mg loading dose), 30 mg, or 10 mg for 24 weeks. Active lesions showed greater baseline expression of inflammatory/immune markers IFNG and CCL5, levels of CD103, and T-cell infiltrates than stable lesions. Patients with more active than stable vitiligo lesions showed higher baseline serum levels of CXCL9 and PD-L1, while patients with more stable than active lesions showed higher baseline serum levels of HO-1. At Week 24, ritlecitinib 50 mg significantly stabilized mean percent change from baseline in depigmentation extent in both active lesions and stable lesions vs. placebo-response, with stable lesions showing greater repigmentation. After 24 weeks of treatment, ritlecitinib 50 mg increased expression of melanocyte markers in stable lesions, while Th1/Th2-related and co-stimulatory molecules decreased significantly in both stable and active lesions. Serum from patients with more active than stable lesions showed decreased levels of ICOS and NK cell activation markers. These data, confirmed at transcription/protein levels, indicate that stable lesion repigmentation occurs early with ritlecitinib, while active lesions require stabilization of inflammation first. ClinicalTrials.gov: NCT03715829.
Keywords: Biomarkers; JAK inhibitor; Non-segmental vitiligo; Ritlecitinib; Vitiligo.
© 2024. The Author(s).
Conflict of interest statement
Y. Yamaguchi, E. Peeva, L.A. Cox, A. Banerjee, and A. Sloan are employees of Pfizer, or were employees of Pfizer when this analysis was conducted and may hold stock and/or stock options with Pfizer. R. Shore declares serving as an investigator and consultant for Pfizer, and as a consultant and advisor for 2020 GeneSystems. D. Thaci declares serving as a consultant, investigator, speaker, and participating in scientific advisory boards for AbbVie, Almirall, Amgen, Biogen Idec, Bristol Myers Squibb, Janssen-Cilag, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Samsung, Sanofi, and UCB; and has received research/educational grants from AbbVie, LEO Pharma, and Novartis. A.K. Ganesan declares serving as a consultant for AbbVie, Allergan Aesthetics, and Viela Bio. K. Ezzedine declares serving as a consultant for Incyte, La Roche Posay, Pfizer, Pierre Fabre, Sanofi, and Viela Bio. E. Guttman-Yassky declares being an employee of Mount Sinai receiving research funds (grants paid to the institution) from AbbVie, Almirall, Amgen, AnaptysBio, Asana Biosciences, AstraZeneca, Boehringer Ingelheim, Celgene, Dermavant, DS Biopharma, Eli Lilly, Galderma, Glenmark/Ichnos Sciences, Innovaderm Research, Janssen, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Novartis, Pfizer, Ralexar, Regeneron Pharmaceuticals, Inc., Sienna Biopharma, UCB, and Union Therapeutics/AntibioTx; and is a consultant for AbbVie, Aditum Bio, Almirall, Alpine, Amgen, Arena, Asana Biosciences, AstraZeneca, Bluefin Biomedicine, Boehringer Ingelheim, Boston Pharmaceuticals, Botanix, Bristol Meyers Squibb, Cara Therapeutics, Celgene, Clinical Outcome Solutions, DBV, Dermavant Sciences, Dermira Inc, Douglas Pharmaceutical, DS Biopharma, Eli Lilly, EMD Serono, Evelo Bioscience, Evidera, FIDE, Galderma, GlaxoSmithKline, Haus Bioceuticals, Ichnos Sciences, Incyte, Kyowa Kirin, Larrk Bio, LEO Pharma, Medicxi, Medscape, Neuralstem, Noble Insights, Novan, Novartis, Okava Pharmaceuticals, Pandion Therapeutics, Pfizer, Principia Biopharma, RAPT Therapeutics, Realm, Regeneron Pharmaceuticals Inc, Sanofi, SATO Pharmaceutical, Sienna Biopharma, Seanergy Dermatology, Seelos Therapeutics, Serpin Pharma, Siolta Therapeutics, Sonoma Biotherapeutics, Sun Pharma, Target PharmaSolutions and Union Therapeutics, Vanda Pharmaceuticals, Ventyx Biosciences, and Vimalan. G. Han declares being an investigator for Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, Eli Lilly, Novartis, Janssen, MC2, PellePharm, Pfizer, and UCB; and a consultant, advisor, or speaker for Abbvie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Castle Biosciences, Dermavant, Dermtech, Eli Lilly, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, SUN Pharmaceuticals, and UCB. E. Del Duca and J. Bar declare no interests. P. Facheris has served as a consultant for Eli Lilly. The study is sponsored by Pfizer. Medical writing support was provided by Ellen Mercado, PhD, and Carolyn Maskin, PhD, of Nucleus Global and funded by Pfizer.
Figures




References
-
- Martins C, Migayron L, Drullion C, Jacquemin C, Lucchese F, Rambert J et al (2021) Vitiligo skin t cells are prone to produce type 1 and type 2 cytokines to induce melanocyte dysfunction and epidermal inflammatory response through jak signaling. J Invest Dermatol. 10.1016/j.jid.2021.09.015 10.1016/j.jid.2021.09.015 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

