Respiratory performance in Duchenne muscular dystrophy: Clinical manifestations and lessons from animal models
- PMID: 39023735
- PMCID: PMC11363095
- DOI: 10.1113/EP091967
Respiratory performance in Duchenne muscular dystrophy: Clinical manifestations and lessons from animal models
Abstract
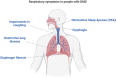
Duchenne muscular dystrophy (DMD) is a fatal genetic neuromuscular disease. Lack of dystrophin in skeletal muscles leads to intrinsic weakness, injury, subsequent degeneration and fibrosis, decreasing contractile function. Dystropathology eventually presents in all inspiratory and expiratory muscles of breathing, severely curtailing their critical function. In people with DMD, premature death is caused by respiratory or cardiac failure. There is an urgent need to develop therapies that improve quality of life and extend life expectancy in DMD. Surprisingly, there is a dearth of information on respiratory control in animal models of DMD, and respiratory outcome measures are often limited or absent in clinical trials. Characterization of respiratory performance in murine and canine models has revealed extensive remodelling of the diaphragm, the major muscle of inspiration. However, significant compensation by extradiaphragmatic muscles of breathing is evident in early disease, contributing to preservation of peak respiratory system performance. Loss of compensation afforded by accessory muscles in advanced disease is ultimately associated with compromised respiratory performance. A new and potentially more translatable murine model of DMD, the D2.mdx mouse, has recently been developed. Respiratory performance in D2.mdx mice is yet to be characterized fully. However, based on histopathological features, D2.mdx mice might serve as useful preclinical models, facilitating the testing of new therapeutics that rescue respiratory function. This review summarizes the pathophysiological mechanisms associated with DMD both in humans and in animal models, with a focus on breathing. We consider the translational value of each model to human DMD and highlight the urgent need for comprehensive characterization of breathing in representative preclinical models to better inform human trials.
Keywords: D2.mdx mice; Duchenne muscular dystrophy; control of breathing; mdx mice; peak inspiratory pressure.
© 2024 The Author(s). Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures





Similar articles
-
Obligatory and accessory respiratory muscle structure, function and control in early and advanced disease in the mdx mouse model of Duchenne muscular dystrophy.J Physiol. 2025 Jul;603(14):4113-4152. doi: 10.1113/JP288709. Epub 2025 Jun 18. J Physiol. 2025. PMID: 40532105 Free PMC article.
-
Loss of compensation afforded by accessory muscles of breathing leads to respiratory system compromise in the mdx mouse model of Duchenne muscular dystrophy.J Physiol. 2023 Oct;601(19):4441-4467. doi: 10.1113/JP285203. Epub 2023 Sep 8. J Physiol. 2023. PMID: 37688347
-
Inspiratory pressure-generating capacity is preserved during ventilatory and non-ventilatory behaviours in young dystrophic mdx mice despite profound diaphragm muscle weakness.J Physiol. 2019 Feb;597(3):831-848. doi: 10.1113/JP277443. Epub 2019 Jan 13. J Physiol. 2019. PMID: 30570134 Free PMC article.
-
Breathing with neuromuscular disease: Does compensatory plasticity in the motor drive to breathe offer a potential therapeutic target in muscular dystrophy?Respir Physiol Neurobiol. 2019 Jul;265:49-54. doi: 10.1016/j.resp.2018.06.009. Epub 2018 Jun 19. Respir Physiol Neurobiol. 2019. PMID: 29933052 Review.
-
Pharmacologic management of Duchenne muscular dystrophy: target identification and preclinical trials.ILAR J. 2014;55(1):119-49. doi: 10.1093/ilar/ilu011. ILAR J. 2014. PMID: 24936034 Free PMC article. Review.
Cited by
-
Evaluation of Creatine Monohydrate Supplementation on the Gastrocnemius Muscle of Mice with Muscular Dystrophy: A Preliminary Study.Pathophysiology. 2025 Jan 6;32(1):2. doi: 10.3390/pathophysiology32010002. Pathophysiology. 2025. PMID: 39846639 Free PMC article.
-
Obligatory and accessory respiratory muscle structure, function and control in early and advanced disease in the mdx mouse model of Duchenne muscular dystrophy.J Physiol. 2025 Jul;603(14):4113-4152. doi: 10.1113/JP288709. Epub 2025 Jun 18. J Physiol. 2025. PMID: 40532105 Free PMC article.
-
Effort-independent respiratory monitoring in Duchenne muscular dystrophy: clinical value of impulse oscillometry.Eur J Pediatr. 2025 Aug 20;184(9):565. doi: 10.1007/s00431-025-06377-1. Eur J Pediatr. 2025. PMID: 40833624
References
-
- Allen, R. E. , & Boxhorn, L. K. (1989). Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor‐beta, insulin‐like growth factor I, and fibroblast growth factor. Journal of Cellular Physiology, 138(2), 311–315. - PubMed
-
- Amancio, G. , Grabe‐Guimarães, A. , Haikel, D. , Moreau, J. , Barcellos, N. M. S. , Lacampagne, A. , Matecki, S. , & Cazorla, O. (2017). Effect of pyridostigmine on in vivo and in vitro respiratory muscle of mdx mice. Respiratory Physiology & Neurobiology, 243, 107–114. - PubMed
-
- Anwar, S. , & Yokota, T. (2020). Golodirsen for Duchenne muscular dystrophy. Drugs Today, 56(8), 491. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
