The Principle of Cortical Development and Evolution
- PMID: 39023844
- PMCID: PMC11876516
- DOI: 10.1007/s12264-024-01259-2
The Principle of Cortical Development and Evolution
Erratum in
-
Correction to: The Principle of Cortical Development and Evolution.Neurosci Bull. 2025 Mar;41(3):546. doi: 10.1007/s12264-024-01282-3. Neurosci Bull. 2025. PMID: 39141312 Free PMC article. No abstract available.
Abstract
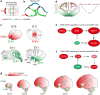
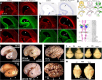
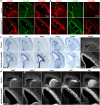
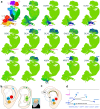
Human's robust cognitive abilities, including creativity and language, are made possible, at least in large part, by evolutionary changes made to the cerebral cortex. This paper reviews the biology and evolution of mammalian cortical radial glial cells (primary neural stem cells) and introduces the concept that a genetically step wise process, based on a core molecular pathway already in use, is the evolutionary process that has molded cortical neurogenesis. The core mechanism, which has been identified in our recent studies, is the extracellular signal-regulated kinase (ERK)-bone morphogenic protein 7 (BMP7)-GLI3 repressor form (GLI3R)-sonic hedgehog (SHH) positive feedback loop. Additionally, I propose that the molecular basis for cortical evolutionary dwarfism, exemplified by the lissencephalic mouse which originated from a larger gyrencephalic ancestor, is an increase in SHH signaling in radial glia, that antagonizes ERK-BMP7 signaling. Finally, I propose that: (1) SHH signaling is not a key regulator of primate cortical expansion and folding; (2) human cortical radial glial cells do not generate neocortical interneurons; (3) human-specific genes may not be essential for most cortical expansion. I hope this review assists colleagues in the field, guiding research to address gaps in our understanding of cortical development and evolution.
Keywords: BMP7; Cortical evolution; Cortical expansion; Cortical gliogenesis; Cortical neurogenesis; FGF-ERK signaling; Human-specific gene; Interneuron; Radial glia; SHH signaling.
© 2024. The Author(s).
Conflict of interest statement
Conflict of interest: The author claims that there are no conflicts of interest.
Figures





References
-
- Rakic P. A century of progress in corticoneurogenesis: From silver impregnation to genetic engineering. Cereb Cortex 2006, 16: i3–i17. - PubMed
-
- Hoch RV, Rubenstein JLR, Pleasure S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol 2009, 20: 378–386. - PubMed
-
- Fernandes M, Hébert JM. The ups and Downs of holoprosencephaly: Dorsal versus ventral patterning forces. Clin Genet 2008, 73: 413–423. - PubMed
-
- Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci 2003, 26: 355–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

