The Penn Electrophysiology of Encoding and Retrieval Study
- PMID: 39023975
- PMCID: PMC12161444
- DOI: 10.1037/xlm0001319
The Penn Electrophysiology of Encoding and Retrieval Study
Abstract
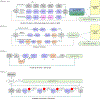
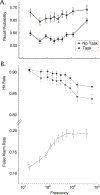
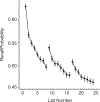
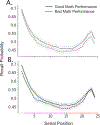
The Penn Electrophysiology of Encoding and Retrieval Study (PEERS) aimed to characterize the behavioral and electrophysiological (EEG) correlates of memory encoding and retrieval in highly practiced individuals. Across five PEERS experiments, 300+ subjects contributed more than 7,000 memory testing sessions with recorded EEG data. Here we tell the story of PEERS: its genesis, evolution, major findings, and the lessons it taught us about taking a big scientific approach in studying memory and the human brain. (PsycInfo Database Record (c) 2024 APA, all rights reserved).
Figures
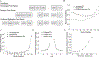
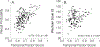





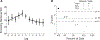
Similar articles
-
Neural correlates of memory in a naturalistic spatiotemporal context.J Exp Psychol Learn Mem Cogn. 2024 Sep;50(9):1404-1420. doi: 10.1037/xlm0001341. J Exp Psychol Learn Mem Cogn. 2024. PMID: 39418452
-
Decoding the tradeoff between encoding and retrieval to predict memory for overlapping events.Neuroimage. 2019 Nov 1;201:116001. doi: 10.1016/j.neuroimage.2019.07.014. Epub 2019 Jul 9. Neuroimage. 2019. PMID: 31299369 Free PMC article.
-
Retrieval practice enhances near but not far transfer of spatial memory.J Exp Psychol Learn Mem Cogn. 2020 Jan;46(1):24-45. doi: 10.1037/xlm0000710. Epub 2019 Apr 18. J Exp Psychol Learn Mem Cogn. 2020. PMID: 30998073
-
Electrophysiological measures of episodic memory control and memory retrieval.Clin EEG Neurosci. 2006 Oct;37(4):315-21. doi: 10.1177/155005940603700409. Clin EEG Neurosci. 2006. PMID: 17073170 Review.
-
Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval.Neuroimage. 2014 Jan 15;85 Pt 2(0 2):721-9. doi: 10.1016/j.neuroimage.2013.08.003. Epub 2013 Aug 8. Neuroimage. 2014. PMID: 23933041 Free PMC article. Review.
Cited by
-
Neural temporal context reinstatement of event structure during memory recall.J Exp Psychol Gen. 2023 Jul;152(7):1840-1872. doi: 10.1037/xge0001354. Epub 2023 Apr 10. J Exp Psychol Gen. 2023. PMID: 37036669 Free PMC article.
-
A context-based model of collaborative inhibition during memory search.Sci Rep. 2024 Nov 12;14(1):27645. doi: 10.1038/s41598-024-78517-w. Sci Rep. 2024. PMID: 39532935 Free PMC article.
-
Neural biomarkers of age-related memory change.Psychol Aging. 2025 May;40(3):265-277. doi: 10.1037/pag0000876. Epub 2025 Feb 6. Psychol Aging. 2025. PMID: 39913469
-
Interresponse times in free recall.J Exp Psychol Learn Mem Cogn. 2025 Jul 3:10.1037/xlm0001498. doi: 10.1037/xlm0001498. Online ahead of print. J Exp Psychol Learn Mem Cogn. 2025. PMID: 40608468
References
-
- Appelhoff S, Sanderson M, Brooks TL, van Vliet M, Quentin R, Holdgraf C, Chaumon M, Mikulan E, Tavabi K, Höchenberger R, Welke D, Brunner C, Rockhill AP, Larson E, Gramfort A, & Jas M (2019). MNE-BIDS: Organizing electrophysiological data into the BIDS format and facilitating their analysis. Journal of Open Source Software, 4(44), Article 1896. 10.21105/joss.01896 - DOI - PMC - PubMed
-
- Balota DA, & Neely JH (1980). Test-expectancy and word-frequency effects in recall and recognition. Journal of Experimental Psychology: Human Learning & Memory, 6(5), 576–587. 10.1037/0278-7393.6.5.576 - DOI
-
- Berger H (1929). Über das elektrenkephalogramm des menschen [On the human electroencephalogram]. Archiv für Psychiatry und Nervenkrankheiten, 87(1), 527–570. 10.1007/BF01797193 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

