Development of PainFace software to simplify, standardize, and scale up mouse grimace analyses
- PMID: 39024163
- PMCID: PMC11287051
- DOI: 10.1097/j.pain.0000000000003187
Development of PainFace software to simplify, standardize, and scale up mouse grimace analyses
Abstract
Facial grimacing is used to quantify spontaneous pain in mice and other mammals, but scoring relies on humans with different levels of proficiency. Here, we developed a cloud-based software platform called PainFace ( http://painface.net ) that uses machine learning to detect 4 facial action units of the mouse grimace scale (orbitals, nose, ears, whiskers) and score facial grimaces of black-coated C57BL/6 male and female mice on a 0 to 8 scale. Platform accuracy was validated in 2 different laboratories, with 3 conditions that evoke grimacing-laparotomy surgery, bilateral hindpaw injection of carrageenan, and intraplantar injection of formalin. PainFace can generate up to 1 grimace score per second from a standard 30 frames/s video, making it possible to quantify facial grimacing over time, and operates at a speed that scales with computing power. By analyzing the frequency distribution of grimace scores, we found that mice spent 7x more time in a "high grimace" state following laparotomy surgery relative to sham surgery controls. Our study shows that PainFace reproducibly quantifies facial grimaces indicative of nonevoked spontaneous pain and enables laboratories to standardize and scale-up facial grimace analyses.
Copyright © 2024 International Association for the Study of Pain.
Conflict of interest statement
Conflict of Interest
RPP is the owner of HypothesisToHardware, LLC. This information was disclosed to UNC Chapel Hill and no conflict of interest was determined.
Figures


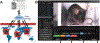


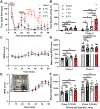
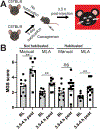
References
-
- Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain 2004;5(9):491–497. - PubMed
-
- Bohic M, Pattison LA, Jhumka ZA, Rossi H, Thackray JK, Ricci M, Foster W, Arnold J, Mossazghi N, Tischfield MA, Yttri EA, Smith ESJ, Abdus-Saboor I, Abraira VE. Mapping the signatures of inflammatory pain and its relief. bioRxiv 2021:2021.2006.2016.448689.
-
- Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol 2009;88(3):184–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

