Caspase-4/11 promotes hyperlipidemia and chronic kidney disease-accelerated vascular inflammation by enhancing trained immunity
- PMID: 39024553
- PMCID: PMC11343595
- DOI: 10.1172/jci.insight.177229
Caspase-4/11 promotes hyperlipidemia and chronic kidney disease-accelerated vascular inflammation by enhancing trained immunity
Abstract
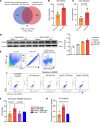
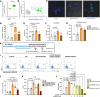
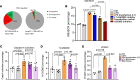
To determine whether hyperlipidemia and chronic kidney disease (CKD) have a synergy in accelerating vascular inflammation via trained immunity (TI), we performed aortic pathological analysis and RNA-Seq of high-fat diet-fed (HFD-fed) 5/6 nephrectomy CKD (HFD+CKD) mice. We made the following findings: (a) HFD+CKD increased aortic cytosolic LPS levels, caspase-11 (CASP11) activation, and 998 gene expressions of TI pathways in the aorta (first-tier TI mechanism); (b) CASP11-/- decreased aortic neointima hyperplasia, aortic recruitment of macrophages, and casp11-gasdermin D-mediated cytokine secretion; (c) CASP11-/- decreased N-terminal gasdermin D (N-GSDMD) membrane expression on aortic endothelial cells and aortic IL-1B levels; (d) LPS transfection into human aortic endothelial cells resulted in CASP4 (human)/CASP11 (mouse) activation and increased N-GSDMD membrane expression; and (e) IL-1B served as the second-tier mechanism underlying HFD+CKD-promoted TI. Taken together, hyperlipidemia and CKD accelerated vascular inflammation by promoting 2-tier trained immunity.
Keywords: Chronic kidney disease; Inflammation; Vascular biology; Vasculitis.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

