Multi-organ immune-related adverse events from immune checkpoint inhibitors and their downstream implications: a retrospective multicohort study
- PMID: 39025103
- PMCID: PMC11316445
- DOI: 10.1016/S1470-2045(24)00278-X
Multi-organ immune-related adverse events from immune checkpoint inhibitors and their downstream implications: a retrospective multicohort study
Abstract
Background: Understanding co-occurrence patterns and prognostic implications of immune-related adverse events is crucial for immunotherapy management. However, previous studies have been limited by sample size and generalisability. In this study, we leveraged a multi-institutional cohort and a population-level database to investigate co-occurrence patterns of and survival outcomes after multi-organ immune-related adverse events among recipients of immune checkpoint inhibitors.
Methods: In this retrospective study, we identified individuals who received immune checkpoint inhibitors between May 31, 2015, and June 29, 2022, from the Massachusetts General Hospital, Brigham and Women's Hospital, and Dana-Farber Cancer Institute (Boston, MA, USA; MGBD cohort), and between April 30, 2010, and Oct 11, 2021, from the independent US population-based TriNetX network. We identified recipients from all datasets using medication codes and names of seven common immune checkpoint inhibitors, and patients were excluded from our analysis if they had incomplete information (eg, diagnosis and medication records) or if they initiated immune checkpoint inhibitor therapy after Oct 11, 2021. Eligible patients from the MGBD cohort were then propensity score matched with recipients of immune checkpoint inhibitors from the TriNetX database (1:2) based on demographic, cancer, and immune checkpoint inhibitor characteristics to facilitate cohort comparability. We applied immune-related adverse event identification rules to identify patients who did and did not have immune-related adverse events in the matched cohorts. To reduce the likelihood of false positives, patients diagnosed with suspected immune-related adverse events within 3 months after chemotherapy were excluded. We performed pairwise correlation analyses, non-negative matrix factorisation, and hierarchical clustering to identify co-occurrence patterns in the MGBD cohort. We conducted landmark overall survival analyses for patient clusters based on predominant immune-related adverse event factors and calculated accompanying hazard ratios (HRs) and 95% CIs, focusing on the 6-month landmark time for primary analyses. We validated our findings using the TriNetX cohort.
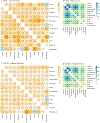
Findings: We identified 15 246 recipients of immune checkpoint inhibitors from MGBD and 50 503 from TriNetX, of whom 13 086 from MGBD and 26 172 from TriNetX were included in our propensity score-matched cohort. Median follow-up durations were 317 days (IQR 113-712) in patients from MGBD and 249 days (91-616) in patients from TriNetX. After applying immune-related adverse event identification rules, 8704 recipients of immune checkpoint inhibitors were retained from MGBD, of whom 3284 (37·7%) had and 5420 (62·3%) did not have immune-related adverse events, and 18 162 recipients were retained from TriNetX, of whom 5538 (30·5%) had and 12 624 (69·5%) did not have immune-related adverse events. In both cohorts, positive pairwise correlations of immune-related adverse events were commonly observed. Co-occurring immune-related adverse events were decomposed into seven factors across organs, revealing seven distinct patient clusters (endocrine, cutaneous, respiratory, gastrointestinal, hepatic, musculoskeletal, and neurological). In the MGBD cohort, the patient clusters that predominantly had endocrine (HR 0·53 [95% CI 0·40-0·70], p<0·0001) and cutaneous (0·61 [0·46-0·81], p=0·0007) immune-related adverse events had favourable overall survival outcomes at the 6-month landmark timepoint, while the other clusters either had unfavourable (respiratory: 1·60 [1·25-2·03], p=0·0001) or neutral survival outcomes (gastrointestinal: 0·86 [0·67-1·10], p=0·23; musculoskeletal: 0·97 [0·78-1·21], p=0·78; hepatic: 1·20 [0·91-1·59], p=0·19; and neurological: 1·30 [0·97-1·74], p=0·074). Similar results were found in the TriNetX cohort (endocrine: HR 0·75 [95% CI 0·60-0·93], p=0·0078; cutaneous: 0·62 [0·48-0·82], p=0·0007; respiratory: 1·21 [1·00-1·46], p=0·044), except for the neurological cluster having unfavourable (rather than neutral) survival outcomes (1·30 [1·06-1·59], p=0·013).
Interpretation: Reliably identifying the immune-related adverse event cluster to which a patient belongs can provide valuable clinical information for prognosticating outcomes of immunotherapy. These insights can be leveraged to counsel patients on the clinical impact of their individual constellation of immune-related adverse events and ultimately develop more personalised surveillance and mitigation strategies.
Funding: US National Institutes of Health.
Copyright © 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests YRS is an advisory board member or consultant and has received honoraria from Pfizer, Incyte Corporation, Sanofi, Galderma, Castle Biosciences, and Iovance Biotherapeutics. K-HY has received consulting fees or honoraria from Curatio DL, Cedars-Sinai Medical Center, Mayo Clinic, Roswell Park Comprehensive Cancer Center, Harvard Medical School, Academia Sinica, Taipei Medical University, and Takeda. NRL is a consultant and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, and Synox Therapeutics. All other authors declare no competing interests.
Figures

References
-
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019. Sep;16(9):563–80. - PubMed
-
- Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: A pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021. Apr 15;53(2):339–54. - PMC - PubMed
-
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021. Dec 20;39(36):4073–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

