Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis
- PMID: 39025226
- PMCID: PMC11832458
- DOI: 10.1016/j.trsl.2024.07.003
Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis
Abstract
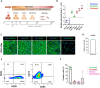
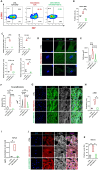
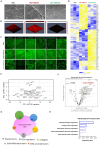
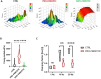
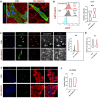
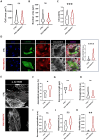
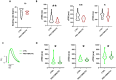
Cardiac fibrosis occurs following insults to the myocardium and is characterized by the abnormal accumulation of non-compliant extracellular matrix (ECM), which compromises cardiomyocyte contractile activity and eventually leads to heart failure. This phenomenon is driven by the activation of cardiac fibroblasts (cFbs) to myofibroblasts and results in changes in ECM biochemical, structural and mechanical properties. The lack of predictive in vitro models of heart fibrosis has so far hampered the search for innovative treatments, as most of the cellular-based in vitro reductionist models do not take into account the leading role of ECM cues in driving the progression of the pathology. Here, we devised a single-step decellularization protocol to obtain and thoroughly characterize the biochemical and micro-mechanical properties of the ECM secreted by activated cFbs differentiated from human induced pluripotent stem cells (iPSCs). We activated iPSC-derived cFbs to the myofibroblast phenotype by tuning basic fibroblast growth factor (bFGF) and transforming growth factor beta 1 (TGF-β1) signalling and confirmed that activated cells acquired key features of myofibroblast phenotype, like SMAD2/3 nuclear shuttling, the formation of aligned alpha-smooth muscle actin (α-SMA)-rich stress fibres and increased focal adhesions (FAs) assembly. Next, we used Mass Spectrometry, nanoindentation, scanning electron and confocal microscopy to unveil the characteristic composition and the visco-elastic properties of the abundant, collagen-rich ECM deposited by cardiac myofibroblasts in vitro. Finally, we demonstrated that the fibrotic ECM activates mechanosensitive pathways in iPSC-derived cardiomyocytes, impacting on their shape, sarcomere assembly, phenotype, and calcium handling properties. We thus propose human bio-inspired decellularized matrices as animal-free, isogenic cardiomyocyte culture substrates recapitulating key pathophysiological changes occurring at the cellular level during cardiac fibrosis.
Keywords: Cardiac fibrosis modelling; Decellularized extracellular matrix; Induced pluripotent stem cells; iPSC-derived-cardiac fibroblasts; iPSC-derived-cardiomyocytes.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Luca Bersanini and Malin Becker are employees of Optics11 Life B.V.
Figures
References
-
- Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Research. 2016 https://f1000research.com/articles/5-752 [cited 2023 Oct 31]. Available from: - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

