Sphinganine recruits TLR4 adaptors in macrophages and promotes inflammation in murine models of sepsis and melanoma
- PMID: 39025856
- PMCID: PMC11258287
- DOI: 10.1038/s41467-024-50341-w
Sphinganine recruits TLR4 adaptors in macrophages and promotes inflammation in murine models of sepsis and melanoma
Abstract
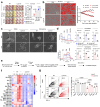
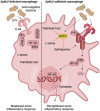
After recognizing its ligand lipopolysaccharide, Toll-like receptor 4 (TLR4) recruits adaptor proteins to the cell membrane, thereby initiating downstream signaling and triggering inflammation. Whether this recruitment of adaptor proteins is dependent solely on protein-protein interactions is unknown. Here, we report that the sphingolipid sphinganine physically interacts with the adaptor proteins MyD88 and TIRAP and promotes MyD88 recruitment in macrophages. Myeloid cell-specific deficiency in serine palmitoyltransferase long chain base subunit 2, which encodes the key enzyme catalyzing sphingolipid biosynthesis, decreases the membrane recruitment of MyD88 and inhibits inflammatory responses in in vitro bone marrow-derived macrophage and in vivo sepsis models. In a melanoma mouse model, serine palmitoyltransferase long chain base subunit 2 deficiency decreases anti-tumor myeloid cell responses and increases tumor growth. Therefore, sphinganine biosynthesis is required for the initiation of TLR4 signal transduction and serves as a checkpoint for macrophage pattern recognition in sepsis and melanoma mouse models.
© 2024. The Author(s).
Conflict of interest statement
G.C. receives research funding from Bayer AG and Boehringer Ingelheim, which has no direct relevance to the findings presented in this study. The remaining authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

