Ezrin, radixin, and moesin are dispensable for macrophage migration and cellular cortex mechanics
- PMID: 39026000
- PMCID: PMC11535515
- DOI: 10.1038/s44318-024-00173-7
Ezrin, radixin, and moesin are dispensable for macrophage migration and cellular cortex mechanics
Abstract
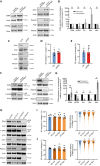
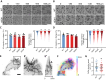
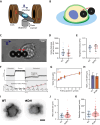
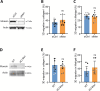
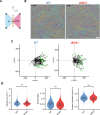
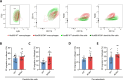
The cellular cortex provides crucial mechanical support and plays critical roles during cell division and migration. The proteins of the ERM family, comprised of ezrin, radixin, and moesin, are central to these processes by linking the plasma membrane to the actin cytoskeleton. To investigate the contributions of the ERM proteins to leukocyte migration, we generated single and triple ERM knockout macrophages. Surprisingly, we found that even in the absence of ERM proteins, macrophages still form the different actin structures promoting cell migration, such as filopodia, lamellipodia, podosomes, and ruffles. Furthermore, we discovered that, unlike every other cell type previously investigated, the single or triple knockout of ERM proteins does not affect macrophage migration in diverse contexts. Finally, we demonstrated that the loss of ERMs in macrophages does not affect the mechanical properties of their cortex. These findings challenge the notion that ERMs are universally essential for cortex mechanics and cell migration and support the notion that the macrophage cortex may have diverged from that of other cells to allow for their uniquely adaptive cortical plasticity.
Keywords: Cell Cortex; Cell Migration; Cytoskeleton; ERM; Macrophages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Accarias S, Sanchez T, Labrousse A, Ben-Neji M, Boyance A, Poincloux R, Maridonneau-Parini I, Le Cabec V (2020) Genetic engineering of hoxb8 immortalized hematopoietic progenitors: a potent tool to study macrophage tissue migration. J Cell Sci 133:jcs236703 - PubMed
-
- Akisawa N, Nishimori I, Iwamura T, Onishi S, Hollingsworth MA (1999) High levels of ezrin expressed by human pancreatic adenocarcinoma cell lines with high metastatic potential. Biochem Biophys Res Commun 258:395–400 - PubMed
-
- Barik GK, Sahay O, Paul D, Santra MK (2022) Ezrin gone rogue in cancer progression and metastasis: An enticing therapeutic target. Biochim Biophys Acta Rev Cancer 1877:188753 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

