Amyloid-β peptide signature associated with cerebral amyloid angiopathy in familial Alzheimer's disease with APPdup and Down syndrome
- PMID: 39026031
- PMCID: PMC11258176
- DOI: 10.1007/s00401-024-02756-4
Amyloid-β peptide signature associated with cerebral amyloid angiopathy in familial Alzheimer's disease with APPdup and Down syndrome
Abstract
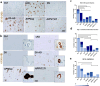
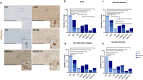
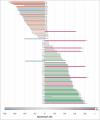
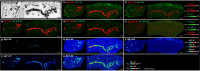
Alzheimer's disease (AD) is characterized by extracellular amyloid plaques containing amyloid-β (Aβ) peptides, intraneuronal neurofibrillary tangles, extracellular neuropil threads, and dystrophic neurites surrounding plaques composed of hyperphosphorylated tau protein (pTau). Aβ can also deposit in blood vessel walls leading to cerebral amyloid angiopathy (CAA). While amyloid plaques in AD brains are constant, CAA varies among cases. The study focuses on differences observed between rare and poorly studied patient groups with APP duplications (APPdup) and Down syndrome (DS) reported to have higher frequencies of elevated CAA levels in comparison to sporadic AD (sAD), most of APP mutations, and controls. We compared Aβ and tau pathologies in postmortem brain tissues across cases and Aβ peptides using mass spectrometry (MS). We further characterized the spatial distribution of Aβ peptides with MS-brain imaging. While intraparenchymal Aβ deposits were numerous in sAD, DS with AD (DS-AD) and AD with APP mutations, these were less abundant in APPdup. On the contrary, Aβ deposits in the blood vessels were abundant in APPdup and DS-AD while only APPdup cases displayed high Aβ deposits in capillaries. Investigation of Aβ peptide profiles showed a specific increase in Aβx-37, Aβx-38 and Aβx-40 but not Aβx-42 in APPdup cases and to a lower extent in DS-AD cases. Interestingly, N-truncated Aβ2-x peptides were particularly increased in APPdup compared to all other groups. This result was confirmed by MS-imaging of leptomeningeal and parenchymal vessels from an APPdup case, suggesting that CAA is associated with accumulation of shorter Aβ peptides truncated both at N- and C-termini in blood vessels. Altogether, this study identified striking differences in the localization and composition of Aβ deposits between AD cases, particularly APPdup and DS-AD, both carrying three genomic copies of the APP gene. Detection of specific Aβ peptides in CSF or plasma of these patients could improve the diagnosis of CAA and their inclusion in anti-amyloid immunotherapy treatments.
Keywords: Alzheimer’s disease; Aβ peptides; Cerebral amyloid angiopathy; Down syndrome; Mass spectrometry; Neuropathology.
© 2024. The Author(s).
Conflict of interest statement
KB has served as a consultant and at advisory boards for Acumen, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). AS has served on scientific advisory boards for AC-Immune, and ProMIS Neurosciences.
Figures





References
-
- Antonell A, Gelpi E, Sanchez-Valle R, Martinez R, Molinuevo JL, Llado A. Breakpoint sequence analysis of an AbetaPP locus duplication associated with autosomal dominant Alzheimer's disease and severe cerebral amyloid angiopathy. J Alzheimers Dis. 2012;28:303–308. doi: 10.3233/JAD-2011-110911. - DOI - PubMed
-
- Baksh RA, Pape SE, Chan LF, Aslam AA, Gulliford MC, Strydom A, Consortium G-D Multiple morbidity across the lifespan in people with Down syndrome or intellectual disabilities: a population-based cohort study using electronic health records. Lancet Public Health. 2023;8:e453–e462. doi: 10.1016/S2468-2667(23)00057-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

