Precision imaging of cardiac function and scar size in acute and chronic porcine myocardial infarction using ultrahigh-field MRI
- PMID: 39026075
- PMCID: PMC11258271
- DOI: 10.1038/s43856-024-00559-y
Precision imaging of cardiac function and scar size in acute and chronic porcine myocardial infarction using ultrahigh-field MRI
Abstract
Background: 7 T cardiac magnetic resonance imaging (MRI) studies may enable higher precision in clinical metrics like cardiac function, ventricular mass, and more. Higher precision may allow early detection of functional impairment and early evaluation of treatment responses in clinical practice and pre-clinical studies.
Methods: Seven female German Landrace pigs were scanned prior to and at three time points (3-4 days, 7-10 days, and ~60 days) post myocardial infarction using a whole body 7 T system and three radiofrequency (RF) coils developed and built in-house to accompany animal growth.
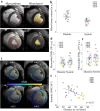
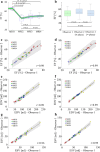
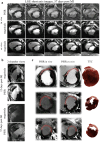
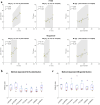
Results: The combination of dedicated RF hardware and 7 T MRI enables a longitudinal study in a pig model of acute and chronic infarction, providing consistent blood tissue contrast and high signal-to-noise ratio (SNR) in measurements of cardiac function, as well as low coefficients of variation (CoV) for ejection fraction (CoVintra-observer: 2%, CoVinter-observer: 3.8%) and infarct size (CoVintra-observer: 8.4%, CoVinter-observer: 3.8%), despite drastic animal growth.
Conclusions: Best results are achieved via manual segmentation. We define state-of-the-art procedures for large animal studies at 7 T.
Plain language summary
In magnetic resonance imaging (MRI), scanners use magnets to generate detailed images of structures in the body, such as the heart. Stronger magnets can produce stronger magnetic fields, which can be leveraged for better image quality and developing new methods for disease diagnosis. In clinical practice, such systems using strong magnets are not yet used for imaging of the heart and some safety aspects remain challenging. We apply such an imaging approach in pigs, in which heart structure and function are similar to humans. We focus on the most important clinical imaging aspects following a heart attack, namely heart function and scar detection. We demonstrate that the high magnetic strength system enabled consistent image quality and accuracy. These findings may help to guide future developments in MRI of the heart, for example in patients who have had a heart attack.
© 2024. The Author(s).
Conflict of interest statement
L.M.S. receives research support by Siemens Healthineers. The position of D.L. is partially paid using this research support. Other authors have no competing interests to declare.
Figures




Similar articles
-
Positron emission tomography for the assessment of myocardial viability: an evidence-based analysis.Ont Health Technol Assess Ser. 2010;10(16):1-80. Epub 2010 Jul 1. Ont Health Technol Assess Ser. 2010. PMID: 23074393 Free PMC article.
-
Magnetic resonance imaging (MRI) for the assessment of myocardial viability: an evidence-based analysis.Ont Health Technol Assess Ser. 2010;10(15):1-45. Epub 2010 Jul 1. Ont Health Technol Assess Ser. 2010. PMID: 23074392 Free PMC article.
-
Submillimeter diffusion tensor imaging and late gadolinium enhancement cardiovascular magnetic resonance of chronic myocardial infarction.J Cardiovasc Magn Reson. 2017 Jan 11;19(1):9. doi: 10.1186/s12968-016-0317-3. J Cardiovasc Magn Reson. 2017. PMID: 28122618 Free PMC article.
-
[Magnetic resonance tomography imaging techniques for diagnosing myocardial vitality].Herz. 1994 Feb;19(1):51-64. Herz. 1994. PMID: 8150414 Review. German.
-
[3 Tesla MRI: successful results with higher field strengths].Radiologe. 2004 Jan;44(1):31-47. doi: 10.1007/s00117-003-1000-x. Radiologe. 2004. PMID: 14997868 Review. German.
Cited by
-
Cardiac MRI of differing ischemia and reperfusion times in a myocardial infarction pig model.Sci Rep. 2025 Jul 19;15(1):26270. doi: 10.1038/s41598-025-11390-3. Sci Rep. 2025. PMID: 40683945 Free PMC article.
-
Ultra-high field cardiac MRI in large animals and humans for translational cardiovascular research.Front Cardiovasc Med. 2023 May 15;10:1068390. doi: 10.3389/fcvm.2023.1068390. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37255709 Free PMC article.
References
-
- Stäb, D. et al. Cardiac magnetic resonance imaging at 7 Tesla. JoVE143, e55853 (2019). - PubMed
LinkOut - more resources
Full Text Sources

