Utilising systematic reviews to assess potential overtreatment and claim for better evidence-based research: an analysis of anticancer drugs versus supportive care in advanced esophageal cancer
- PMID: 39026378
- PMCID: PMC11256491
- DOI: 10.1186/s13643-024-02594-1
Utilising systematic reviews to assess potential overtreatment and claim for better evidence-based research: an analysis of anticancer drugs versus supportive care in advanced esophageal cancer
Abstract
Background: Highlighting the identified gaps in evidence-based research concerning advanced esophageal cancer (EC) treatment and care, this review evaluates the efficacy and safety of anticancer drugs compared to supportive care for advanced EC patients, aiming to assess the appropriateness of usual treatments and identify the gaps that need to be filled with primary research.
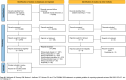
Methods: We searched (May 2022) MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), Epistemonikos, and trial registries (ClinicalTrials.gov and PROSPERO) for randomised controlled trials (RCTs) comparing anticancer drugs (chemotherapy, immunotherapy, or biological/targeted therapy) with supportive care in advanced EC. The results were summarised using GRADE summary of finding tables.
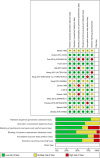
Results: We included 15 RCTs. Most studies did not have a special focus on EC, did not detail the treatment lines in all patients, and did not evaluate all outcomes. Anticancer drugs may result in a slight increase in overall survival (OS) (HR 0.78; 95% CI 0.71, 0.86; MD 0.83 months) and better progression-free survival (PFS) (HR 0.56 95% CI 0.49, 0.64, MD 0.68 months), but also may increase toxicity (RR 1.37; 95% CI 1.13, 1.65), without a significant improvement in quality of life. The certainty of evidence was low or very low due to indirectness of results and lack of specific focus on EC in some studies.
Conclusion: RCTs on advanced EC lack specificity, detailed treatment line information, and evaluation of all relevant outcomes. Moreover, when they find any benefit, this is negligible. Therefore, the certainty to justify anticancer drug treatments instead of supportive care in advanced EC is low or very low, and this information should be actively shared with affected patients. More and better RCTs should be conducted to assess whether any old or new proposed treatment for advanced EC patients provides a better balance of benefits and harms than the supportive care.
Systematic review registration: The study protocol was registered in OSF ( https://doi.org/10.17605/OSF.IO/7CHX6 ) on 2022-03-29.
Keywords: Advanced esophageal cancer; Chemotherapy; Immunotherapy; Meta-analysis; Systematic review; Targeted therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2021. pp. 209–249. 10.3322/caac.21660. - PubMed
-
- Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658.e2. doi: 10.1053/j.gastro.2022.05.054. - DOI - PubMed
-
- Arnold M, Morgan E, Bardot A, Rutherford MJ, Ferlay J, Little A, et al. International variation in oesophageal and gastric cancer survival 2012–2014: differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study) Gut. 2022;71:1532–1543. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

