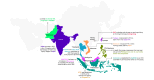
Bridging Real-World Data Gaps: Connecting Dots Across 10 Asian Countries
- PMID: 39026427
- PMCID: PMC11362708
- DOI: 10.2196/58548
Bridging Real-World Data Gaps: Connecting Dots Across 10 Asian Countries
Abstract
The economic trend and the health care landscape are rapidly evolving across Asia. Effective real-world data (RWD) for regulatory and clinical decision-making is a crucial milestone associated with this evolution. This necessitates a critical evaluation of RWD generation within distinct nations for the use of various RWD warehouses in the generation of real-world evidence (RWE). In this article, we outline the RWD generation trends for 2 contrasting nation archetypes: "Solo Scholars"-nations with relatively self-sufficient RWD research systems-and "Global Collaborators"-countries largely reliant on international infrastructures for RWD generation. The key trends and patterns in RWD generation, country-specific insights into the predominant databases used in each country to produce RWE, and insights into the broader landscape of RWD database use across these countries are discussed. Conclusively, the data point out the heterogeneous nature of RWD generation practices across 10 different Asian nations and advocate for strategic enhancements in data harmonization. The evidence highlights the imperative for improved database integration and the establishment of standardized protocols and infrastructure for leveraging electronic medical records (EMR) in streamlining RWD acquisition. The clinical data analysis and reporting system of Hong Kong is an excellent example of a successful EMR system that showcases the capacity of integrated robust EMR platforms to consolidate and produce diverse RWE. This, in turn, can potentially reduce the necessity for reliance on numerous condition-specific local and global registries or limited and largely unavailable medical insurance or claims databases in most Asian nations. Linking health technology assessment processes with open data initiatives such as the Observational Medical Outcomes Partnership Common Data Model and the Observational Health Data Sciences and Informatics could enable the leveraging of global data resources to inform local decision-making. Advancing such initiatives is crucial for reinforcing health care frameworks in resource-limited settings and advancing toward cohesive, evidence-driven health care policy and improved patient outcomes in the region.
Keywords: Asia; EMR; HTA; electronic medical records; health care databases; health technology assessment; real-world data; real-world evidence.
©Guilherme Silva Julian, Wen-Yi Shau, Hsu-Wen Chou, Sajita Setia. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 15.08.2024.
Conflict of interest statement
Conflicts of Interest: GSJ, H-WC, and W-YS declare that while being employees of Pfizer, there is no conflict of interest in relation to the work presented in this article. The views and opinions expressed herein are solely those of the author(s) and do not reflect the views or positions of their employers.
Figures








References
-
- Naidoo P, Bouharati C, Rambiritch V, Jose N, Karamchand S, Chilton R, Leisegang R. Real-world evidence and product development: opportunities, challenges and risk mitigation. Wien Klin Wochenschr. 2021;133(15-16):840–846. doi: 10.1007/s00508-021-01851-w. http://europepmc.org/abstract/MED/33837463 10.1007/s00508-021-01851-w - DOI - PMC - PubMed
-
- Shau W, Setia S, Shinde S, Santoso H, Furtner D. Generating fit-for-purpose real-world evidence in Asia: how far are we from closing the gaps? Perspect Clin Res. 2023;14(3):108–113. doi: 10.4103/picr.picr_193_22. https://europepmc.org/abstract/MED/37554247 PCR-14-108 - DOI - PMC - PubMed
-
- Bhatt A. Conducting real-world evidence studies in India. Perspect Clin Res. 2019;10(2):51–56. doi: 10.4103/picr.PICR_8_19. http://www.picronline.org/article.asp?issn=2229-3485;year=2019;volume=10... PCR-10-51 - DOI - PMC - PubMed
-
- Shau WY, Setia S, Shinde SP, Santoso H, Furtner D. Contemporary databases in real-world studies regarding the diverse health care systems of India, Thailand, and Taiwan: protocol for a scoping review. JMIR Res Protoc. 2022;11(12):e43741. doi: 10.2196/43741. https://www.researchprotocols.org/2022/12/e43741/ v11i12e43741 - DOI - PMC - PubMed
-
- Comparative effectiveness research. National Library of Medicine, National Center for Biotechnology Information. [2024-02-12]. https://www.ncbi.nlm.nih.gov/mesh/?term=comparative+effectiveness+research/
LinkOut - more resources
Full Text Sources

