ReFerm®: a postbiotic fermented oat gruel composition is reducing mast cell degranulation in the colon of patients with irritable bowel syndrome
- PMID: 39026547
- PMCID: PMC11255971
- DOI: 10.3389/fmed.2024.1408623
ReFerm®: a postbiotic fermented oat gruel composition is reducing mast cell degranulation in the colon of patients with irritable bowel syndrome
Abstract
Background: Irritable bowel syndrome (IBS) is a highly prevalent gastrointestinal disorder that affects ~4% of the global population. ReFerm® is a postbiotic product derived from oat gruel fermented with Lactobacillus plantarum 299v, and it has been shown to have beneficial effects on intestinal permeability in patients with IBS. In this study, we investigated the effects of ReFerm® on regulators of intestinal permeability, namely mast cells and enteric glial cells.
Materials and methods: A total of 30 patients with moderate to severe IBS were treated with an enema containing ReFerm® or a placebo twice daily. The patients underwent sigmoidoscopy with biopsies obtained from the distal colon at baseline and after 14 days of treatment. These biopsies were processed in two ways: some were fixed, embedded in paraffin, sectioned, and stained for mast cells and enteric glial cells; others were cryopreserved, lysed, and subjected to Western blotting to analyze the same markers.
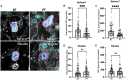
Results: Treatment with ReFerm®, but not the placebo, significantly reduced mast cell tryptase protein levels in the biopsy lysates. Although the number of mast cells remained unchanged in colonic biopsies, ReFerm® treatment significantly reduced mast cell degranulation, a result not observed in the placebo group. Neither ReFerm® or placebo treatment had an impact on total protein levels or the number of enteric glial cells in the biopsies.
Conclusion: ReFerm® treatment significantly reduced both total mast cell tryptase levels and the degranulation of mast cells in colonic biopsies from patients with IBS, suggesting a decrease in mast cell activity as a potential mechanism underlying the beneficial effects of ReFerm®. However, further research is required to assess the molecular mechanisms through which ReFerm® operates in the colons of patients with IBS.
Clinical trial registration: https://clinicaltrials.gov, identifier: NCT05475314.
Keywords: enteric nervous system; functional gastrointestinal disorder; intestinal permeability; mucosal immunology; postbiotics.
Copyright © 2024 Biskou, Walter, Israelsen, Winberg, Bednarska and Keita.
Conflict of interest statement
HI was employed by Nordic Rebalance, which partially financed the study; however, the content of the study was neither influenced nor constrained by this funding. OBi was employed at Linköping University, except for the last 2 months, when she was contracted by Nordic Rebalance as an independent scientific consultant. However, this did not affect or limit the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Medical

