Eligibility of sodium-glucose cotransporter-2 inhibitors in heart failure with preserved ejection fraction: Insights from the Colombian heart failure registry (RECOLFACA)
- PMID: 39027018
- PMCID: PMC11254738
- DOI: 10.1016/j.ijcha.2024.101448
Eligibility of sodium-glucose cotransporter-2 inhibitors in heart failure with preserved ejection fraction: Insights from the Colombian heart failure registry (RECOLFACA)
Abstract
Background: The value of Sodium-glucose cotransporter-2 inhibitors (SGLT-2 inhibitor) therapy in individuals with heart failure with preserved EF (HFpEF) was unknown until the EMPEROR-Preserved trial. We aimed to assess the proportion of patients with HFpEF that are eligible for empagliflozin therapy within the Colombian Heart Failure Registry (RECOLFACA).
Methods: RECOLFACA enrolled adult patients with a HF diagnosis during 2017-2019 from 60 medical centers in Colombia. Criteria of the EMPEROR-Preserved Trial were used to recruit participants. The main outcome was individual eligibility with N-terminal pro-B-type natriuretic peptide (NT-proBNP) criteria, while the secondary outcome was eligibility without NT-proBNP data.
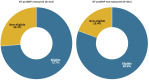
Results: RECOLFACA had 799 patients with HFpEF (mean age70.7 ± 13.5; 50.7 % males). According to the major selection criteria of the EMPEROR Preserved Trial, 73.7 % patients would be eligible for empagliflozin therapy initiation when considering the NT-proBNP threshold. The NT-proBNP threshold represented the main determinant of ineligibility in patients with this biomarker measure (13.6 %; n = 16). In patients without NT-proBNP data, the main reasons for exclusion were the diagnosis of symptomatic hypotension or a systolic blood pressure below 100 mmHg (7.5 %), having an eGFR < 20 ml/min/1.73 m2 (4.3 %), and haemoglobin < 9 g/dl (3.1 %). Excluding NT-proBNP criteria increased empagliflozin eligibility to 80.6 %.
Conclusion: Most patients with HFpEF from RECOLFACA are potential candidates for empagliflozin therapy initiation according to the EMPEROR-Preserved trial criteria. These findings favor the utilization of SGLT-2 inhibitor medications in daily medical practice, which may further decrease morbidity and mortality in HF patients, regardless of their EF classification.
Keywords: Colombia; Ejection fraction; Empagliflozin; Heart failure; SGLT-2 inhibitor.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Fonarow G.C., Stough W.G., Abraham W.T., Albert N.M., Gheorghiade M., Greenberg B.H., et al. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure: A Report From the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. - DOI - PubMed
-
- Lee D.S., Gona P., Vasan R.S., Larson M.G., Benjamin E.J., Wang T.J., et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

