Functional analysis of a novel endo-β-1,6-glucanase Mo Glu16 and its application in detecting cell wall β-1,6-glucan of Magnaporthe oryzae
- PMID: 39027104
- PMCID: PMC11254853
- DOI: 10.3389/fmicb.2024.1429065
Functional analysis of a novel endo-β-1,6-glucanase Mo Glu16 and its application in detecting cell wall β-1,6-glucan of Magnaporthe oryzae
Abstract
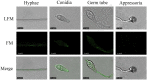
As an essential component of the fungal cell wall, β-1,6-glucan has an important role in the growth and development of fungi, but its distribution has not been investigated in Magnaporthe oryzae. Here, a novel β-1,6-glucanase from M. oryzae, MoGlu16, was cloned and expressed in Pichia pastoris. The enzyme was highly active on pustulan, with a specific activity of 219.0 U/mg at pH 5.0 and 50°C, and showed great selectivity for continuous β-1,6-glycosidic bonding polysaccharides. Based on this, β-1,6-glucan was selectively visualized in the vegetative hyphae, conidia and bud tubes of M. oryzae using a hydrolytically inactive GFP-tagged MoGlu16 with point mutations at the catalytic position (His-MoGlu16E236A-Gfp). The spore germination and appressorium formation were significantly inhibited after incubation of 105/ml conidia with 0.03 μg/μl MoGlu16. Mycelia treated with MoGlu16 produced reactive oxygen species and triggered the cell wall integrity pathway, increasing the expression levels of genes involved in cell wall polysaccharide synthesis. These results revealed that MoGlu16 participated in the remodeling of cell wall in M. oryzae, laying a foundation for the analysis of cell wall structure.
Keywords: Magnaporthe oryzae; cell wall; specificity; β-1,6-glucan; β-1,6-glucanase.
Copyright © 2024 Wang, Li, Li, Cui and Ye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials

