Lipid-nanoparticle-enabled nucleic acid therapeutics for liver disorders
- PMID: 39027251
- PMCID: PMC11252464
- DOI: 10.1016/j.apsb.2024.04.015
Lipid-nanoparticle-enabled nucleic acid therapeutics for liver disorders
Abstract
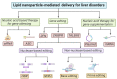
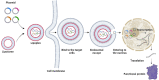
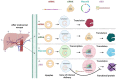
Inherited genetic disorders of the liver pose a significant public health burden. Liver transplantation is often limited by the availability of donor livers and the exorbitant costs of immunosuppressive therapy. To overcome these limitations, nucleic acid therapy provides a hopeful alternative that enables gene repair, gene supplementation, and gene silencing with suitable vectors. Though viral vectors are the most efficient and preferred for gene therapy, pre-existing immunity debilitating immune responses limit their use. As a potential alternative, lipid nanoparticle-mediated vectors are being explored to deliver multiple nucleic acid forms, including pDNA, mRNA, siRNA, and proteins. Herein, we discuss the broader applications of lipid nanoparticles, from protein replacement therapy to restoring the disease mechanism through nucleic acid delivery and gene editing, as well as multiple preclinical and clinical studies as a potential alternative to liver transplantation.
Keywords: ASO; Clinical trials; Gene editing; Gene therapy; Lipid nanoparticle; Liver disorders; Nucleic acid delivery; mRNA; pDNA; siRNA.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics.Acc Chem Res. 2019 Sep 17;52(9):2435-2444. doi: 10.1021/acs.accounts.9b00368. Epub 2019 Aug 9. Acc Chem Res. 2019. PMID: 31397996 Review.
-
Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery.J Control Release. 2022 Jan;341:616-633. doi: 10.1016/j.jconrel.2021.10.031. Epub 2021 Nov 3. J Control Release. 2022. PMID: 34742747 Free PMC article.
-
Novel vectors and approaches for gene therapy in liver diseases.JHEP Rep. 2021 Apr 30;3(4):100300. doi: 10.1016/j.jhepr.2021.100300. eCollection 2021 Aug. JHEP Rep. 2021. PMID: 34159305 Free PMC article. Review.
-
Diosgenin enhances liposome-enabled nucleic acid delivery and CRISPR/Cas9-mediated gene editing by modulating endocytic pathways.Front Bioeng Biotechnol. 2023 Jan 9;10:1031049. doi: 10.3389/fbioe.2022.1031049. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36698628 Free PMC article.
-
Non-viral nucleic acid containing nanoparticles as cancer therapeutics.Expert Opin Drug Deliv. 2016 Oct;13(10):1475-87. doi: 10.1080/17425247.2016.1190707. Epub 2016 Jun 6. Expert Opin Drug Deliv. 2016. PMID: 27248202 Free PMC article. Review.
Cited by
-
Epigenetic editing and epi-drugs: a combination strategy to simultaneously target KDM4 as a novel anticancer approach.Clin Epigenetics. 2025 Jun 19;17(1):105. doi: 10.1186/s13148-025-01913-0. Clin Epigenetics. 2025. PMID: 40537846 Free PMC article.
-
Hepatic Manifestations Following Gene Therapy.Gastro Hep Adv. 2025 Apr 24;4(8):100681. doi: 10.1016/j.gastha.2025.100681. eCollection 2025. Gastro Hep Adv. 2025. PMID: 40520419 Free PMC article. Review.
-
Recent advances in the bench-to-bedside translation of cancer nanomedicines.Acta Pharm Sin B. 2025 Jan;15(1):97-122. doi: 10.1016/j.apsb.2024.12.007. Epub 2024 Dec 14. Acta Pharm Sin B. 2025. PMID: 40041906 Free PMC article. Review.
-
Phospholipid-Drug Conjugates in Cancer Therapy: Emerging Paradigms and Future Directions.AAPS PharmSciTech. 2025 Jul 14;26(6):190. doi: 10.1208/s12249-025-03175-8. AAPS PharmSciTech. 2025. PMID: 40659903 Review.
-
Current Treatment Regimens and Promising Molecular Therapies for Chronic Hepatobiliary Diseases.Biomolecules. 2025 Jan 14;15(1):121. doi: 10.3390/biom15010121. Biomolecules. 2025. PMID: 39858515 Free PMC article. Review.
References
-
- Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. - PubMed
-
- Marcellin P., Kutala B.K. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38:2–6. - PubMed
-
- Ruiz R., Kirk A.D. Long-term toxicity of immunosuppressive therapy. Transplantation of the liver. 2015;97:1354–1363. third edition.
-
- Couvreur P., Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res (N Y) 2006;23:1417–1450. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

