A novel marine-derived anti-acute kidney injury agent targeting peroxiredoxin 1 and its nanodelivery strategy based on ADME optimization
- PMID: 39027260
- PMCID: PMC11252462
- DOI: 10.1016/j.apsb.2024.03.005
A novel marine-derived anti-acute kidney injury agent targeting peroxiredoxin 1 and its nanodelivery strategy based on ADME optimization
Abstract
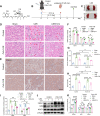
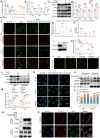
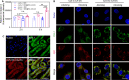
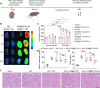
Insufficient therapeutic strategies for acute kidney injury (AKI) necessitate precision therapy targeting its pathogenesis. This study reveals the new mechanism of the marine-derived anti-AKI agent, piericidin glycoside S14, targeting peroxiredoxin 1 (PRDX1). By binding to Cys83 of PRDX1 and augmenting its peroxidase activity, S14 alleviates kidney injury efficiently in Prdx1-overexpression (Prdx1-OE) mice. Besides, S14 also increases PRDX1 nuclear translocation and directly activates the Nrf2/HO-1/NQO1 pathway to inhibit ROS production. Due to the limited druggability of S14 with low bioavailability (2.6%) and poor renal distribution, a pH-sensitive kidney-targeting dodecanamine-chitosan nanoparticle system is constructed to load S14 for precise treatment of AKI. l-Serine conjugation to chitosan imparts specificity to kidney injury molecule-1 (Kim-1)-overexpressed cells. The developed S14-nanodrug exhibits higher therapeutic efficiency by improving the in vivo behavior of S14 significantly. By encapsulation with micelles, the AUC0‒t , half-life time, and renal distribution of S14 increase 2.5-, 1.8-, and 3.1-fold, respectively. The main factors contributing to the improved druggability of S14 nanodrugs include the lower metabolic elimination rate and UDP-glycosyltransferase (UGT)-mediated biotransformation. In summary, this study identifies a new therapeutic target for the marine-derived anti-AKI agent while enhancing its ADME properties and druggability through nanotechnology, thereby driving advancements in marine drug development for AKI.
Keywords: ADME; Acute kidney injury; Druggability; Kim-1 targeted; Marine drug; Nanodrug; Peroxiredoxin 1; Piericidin glycoside.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

