Photophysics and photochemistry of thermally activated delayed fluorescence emitters based on the multiple resonance effect: transient optical and electron paramagnetic resonance studies
- PMID: 39027280
- PMCID: PMC11253189
- DOI: 10.1039/d4sc02513j
Photophysics and photochemistry of thermally activated delayed fluorescence emitters based on the multiple resonance effect: transient optical and electron paramagnetic resonance studies
Abstract
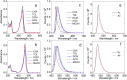
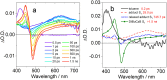
The photochemistry of two representative thermally activated delayed fluorescence (TADF) emitters based on the multiple resonance effect (MRE) (DABNA-1 and DtBuCzB) was studied. No significant TADF was observed in fluid solution, although the compounds have a long-lived triplet state (ca. 30 μs). We found that these planar boron molecules bind with Lewis bases, e.g., 4-dimethylaminopyridine (DMAP) or an N-heterocyclic carbene (NHC). A new blue-shifted absorption band centered at 368 nm was observed for DtBuCzB upon formation of the adduct; however, the fluorescence of the adduct is the same as that of the free DtBuCzB. We propose that photo-dissociation occurs for the DtBuCzB-DMAP adduct, which is confirmed by femtosecond transient absorption spectra, implying that fluorescence originates from DtBuCzB produced by photo-dissociation; the subsequent in situ re-binding was observed with nanosecdon transient absorption spectroscopy. No photo-dissociation was observed for the NHC adduct. Time-resolved electron paramagnetic resonance (TREPR) spectra show that the triplet states of DABNA-1 and DtBuCzB have similar zero field splitting (ZFS) parameters (D = 1450 MHz). Theoretical studies show that the slow ISC is due to small SOC and weak Herzberg-Teller coupling, although the S1/T1 energy gap is small (0.14 eV), which rationalizes the lack of TADF.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










References
LinkOut - more resources
Full Text Sources
Miscellaneous

